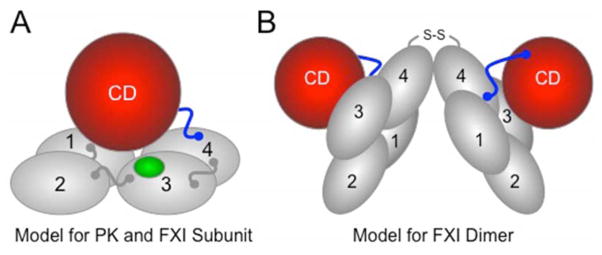
Figure 3. Models for Prekallikrein and Factor XI.
(A) PK and fXI subunit structure. PK and fXI are homologs [31]. Each subunit for these proteins is comprised of four apple domains (1 through 4) and a trypsin-like catalytic domain (CD) [34,35]. The apple domains form a planar disk on which the catalytic domain rests. Activation of PK and fXI by fXIIa occurs by a single proteolytic cleavage within the portion of the proteins indicated in blue. The sequence around the fXIIa cleavage site on fXI differs from that in PK, allowing fXI to also be activated by thrombin [26]. There is a fIX binding site on the fXI apple 3 domain (indicated in green) that is not present on PK [36]. The fIX binding site is not exposed in fXI, but becomes available for fIX binding when fXI is converted to fXIa. (B) The factor XI dimer. FXI is a homodimeric protein in all species in which it has been evaluated [34,35]. The apple 4 domain serves as the interface for two fXI subunits which are oriented at a 70° angle to each other [34] In most species, a disulfide bond (S-S) connects the subunits. While dimeric and monomeric forms of fXI behave similarly in aPTT assays, the monomeric forms perform poorly in mouse thrombosis models, indicating that the dimeric conformation serves a critical function in vivo [29].