Abstract
Transient global ischemia in rodents induces delayed death of hippocampal CA1 neurons, as well as in some hilar neurons of the dentate gyrus, medium aspiny neurons of the striatum, pyramidal neurons in neocortical layers II, V and VI, and Purkinje neurons of the cerebellum. In contrast to focal ischemia that mimics regional stroke in humans, this model of global ischemia mimics the brain injury that occurs after human cardiac arrest. Early events include caspase activation, cleavage of anti-death Bcl-2 family proteins and large mitochondrial channel activity. Genetically engineered mice provide opportunities for study such as the knock-in mouse expressing a caspase-resistant form of Bcl-xL found to exhibit markedly reduced mitochondrial channel activity and reduced vulnerability to ischemia-induced neuronal death1. It is therefore relevant to adapt and develop a simple protocol for producing transient global ischemia in mouse2. The two-vessel occlusion model has been specifically developed to provide optimal outcomes in mouse and offers several advantages over the four-vessel occlusion model traditionally used in rat including the relative ease of the procedure as well as only a single day of surgery. However it should be noted that this procedure has a higher morbidity rate compared to other ischemia models as well as a higher degree of variability. These two disadvantages necessitate the use of a larger cohort of animals, which for many healthy breeding transgenic animals is a non-deterring factor.
Material and Reagents
Eight-week-old C57 mice or a variety of transgenic/knockout mice, body weight 20–25 g
Isoflurane USP (ISOTHESIA) (Butler Schein, catalog number: 029404)
2% lidocaine hydrochloride jelly USP (Healthhotline, catalog number: 1024140)
Analgesic: Flunixiject (Flunixin Meglumine) (Butler Schein, catalog number: 029405)
0.9% normal saline or other vehicle for your reagents
Betadine surgical scrub (7.5% povidone-Iodine) (Healthhotline, catalog number: 6900581)
Sterile ocular lubricant (puralube) (Butler Schein, catalog number: 008897)
Equipment
Anesthesia induction chamber (VetEquip, catalog number: 941444)
Vapomatic anesthetic vaporizer (Bickford)
Dissecting microscope with a surgical board included
Facial mask
Electric shaver
Timer
Q-tips
Gauze
Scissors
Heating lamp
Scalpel handle (Fine Science Tools, catalog number: 10003-12)
Blade #11 (Fine Science Tools, catalog number: 10011-00)
2× tissue forceps (Fine Science Tools, Dumont 5/45, catalog number: 11251-35)
2× Micro serrefine clamp (Fine Science Tools, catalog number: 18055-04)
Forceps style slamp applicator (Fine Science Tools, catalog number: 18057-14)
Hot bead sterilizer (Fine Science Tools, catalog number: 18000-50)
Wound closing Clip-EZ Clip Kit (Stoelting, catalog number: 59020)
Silk surgical suture (Healthhotline, catalog number: 100-5597)
BD 1 ml syringe 26G×3/8 (0.45 mm×10 mm) (Thermo Fischer Scientific, catalog number: 14-823-2E)
Surgical shaver (Stoelting, catalog number: 5148/Blade, Surgical/Shaving, catalog number: 50, mice, Steel, catalog number: 5148)
Procedure
- Anesthesia
-
1Set the mouse in the anesthesia chamber where a flow of Isoflurane 4% in a mixture of N2:O2 (40:30) will be delivered by a tube attached to a Vapomatic Anesthetic Vaporizer (CWE). Mouse is adequately anaesthetized when it fails to twitch upon tail or foot pinch.
-
1
- Animal preparation
-
2Shave the area that will undergo procedure (neck), with a surgical shaver.
-
3Set the mouse on the surgical board (supine position) with the head placed into a facial mask connected to the Vapomatic Anesthetic Vaporizer (CWE) through which a flow of Isoflurane 2% in a mixture of N2:O2 (40:30) is delivered constantly until carotid clamping.
-
4Stabilize the mouse by taping down the forelegs (surgical tape or scotch tape will suffice).
-
2
- Surgical procedure
-
5With a Q-Tip apply Betadine Surgical Scrub to decontaminate the skin.
-
6With a scalpel, make a ventral midline vertical 0.5–1 cm skin incision on the neck (at exactly, and parallel to, the ventral midline).
-
7Lift up fat and connective tissue with forceps and cut horizontally at bottom to enter the tissue plane underneath.
-
8Locate sternocleidomastoid muscle and muscles around the trachea. Open the space between these muscles to expose blood vessels.
-
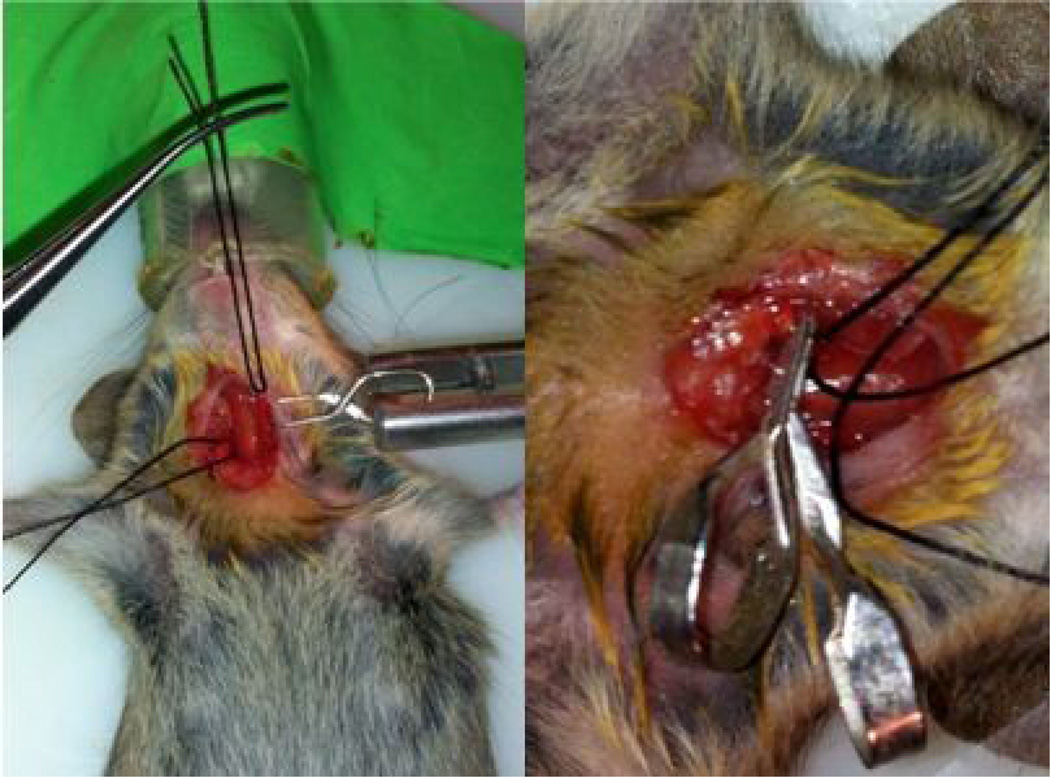
9While observing the field in the microscope, using forceps gently isolate (CRITICAL: Avoid any direct contact with the vagal nerve-this step requires some practice) common carotid artery (CCA) from nerves (vagal nerve etc.) and veins. Nerves are white. The artery is medial to the vagal nerve. Perform this step for both CCAs.
- 10
-
11Expose the CCA by pulling it up through the silk suture (left) and clip both CCAs using micro serrefine clamps (right).
-
12Set timer to 30 min (clamping time may vary depending on laboratory conditions i.e. clamp position and strength; investigators should perform pilot experiments and test times between 20–45* min to obtain optimal ischemia with minimal mortality).
-
13Reduce the isoflurane to 1% (depending on how the animals respond after clamping). The mouse should have no response to tail pinch. Respirations will be variable.
-
14*At 30 min remove clips and silk suture underneath the CCA.
-
15Close the skin incision with autoclips.
-
5
- Check point during clipping:
-
16Diaphragm Contraction (~hiccups); if this happens the vagal nerve has been scratched. If there is vagal damage the animals rarely survive the procedure.
-
16
- Post-operative care:
-
17Give (analgesic) Flunixin Subcutaneously, 2.2 mg/kg.
-
18Apply Lidocaine directly to the incision.
-
19Apply the ocular lubricant to prevent drying of the eyes.
-
20Set the mouse in a recovery cage and keep it warm by using a heating lamp until recovered.
-
21Daily monitoring of the animal’s condition is necessary to determine if there is pain or abnormal behavior.
-
22At desired time points, mouse will be anesthetized and perfused transcardially with saline 0.9%, followed by buffered 4% paraformaldehyde for immunohistochemistry. Note: At 4–5 day post ischemia FluoroJade staining can be used to determine the efficacy of ischemia. It is critical to check the CA1 region of the hippocampus bilaterally as mouse ischemia is not always bilateral. Because of the non-stereotypical vasculature of mice approximately 50% of mice will show histological signs of ischemia with this method.
-
17
Acknowledgments
This work was supported by the NIH (grant number NS045876).
References
- 1.Ofengeim D, Chen YB, Miyawaki T, Li H, Sacchetti S, Flannery RJ, Alavian KN, Pontarelli F, Roelofs BA, Hickman JA, Hardwick JM, Zukin RS, Jonas EA. N-terminally cleaved Bcl-xL mediates ischemia-induced neuronal death. Nat Neurosci. 2012;15(4):574–580. doi: 10.1038/nn.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhen G, Dore S. Optimized protocol to reduce variable outcomes for the bilateral common carotid artery occlusion model in mice. J Neurosci Methods. 2007;166(1):73–80. doi: 10.1016/j.jneumeth.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]