Table 1.
Reaction approach and optimization
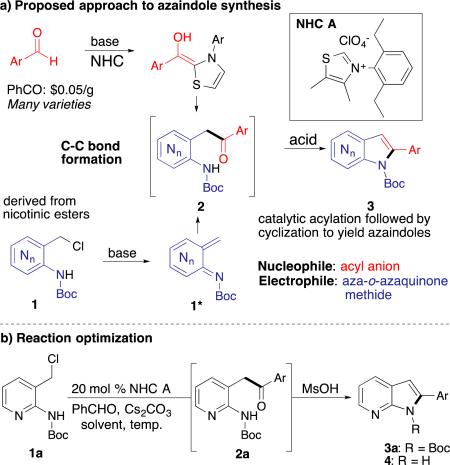
| |||||||
|---|---|---|---|---|---|---|---|
| Entrya | Solvent | % cat. | Equiv MsOH | Temp. (°C) | 2 (%)b | 3 (%)b | 4 (%)b |
| 1 | THF | 20 | - | rt | 78 | - | - |
| 2 | Dioxane | 20 | - | rt | 96 | - | - |
| 3 | Diethyl ether | 20 | - | rt | 59 | - | - |
| 4 | MTBE | 20 | - | rt | 64 | - | - |
| 5 | 2-methyl THF | 20 | - | rt | 66 | - | - |
| 6c | Dioxane | 10 | - | rt | 68 | - | - |
| 7d | Dioxane | 5 | - | rt | trace | - | - |
| 8c | Dioxane | 5 | - | 45 | trace | - | - |
| 9 | THF | 20 | 6.5 | rt | 70 | 0 | 0 |
| 10 | Dioxane | 20 | 6.5 | rt | 30 | 36 | 38 |
| 11e | Dioxane | 20 | 7.5 | rt, then 40 | 0 | 0 | 90 |
optimization reactions conducted on a 0.04 mmol scale (0.1 M) using 20% catalyst, 1.2 equivalents of aldehyde, and 1.2 equivalents of Cs2CO3 unless otherwise noted.
yield of isolated material
conducted at 0.2 M concentration
conducted at 0.4 M concentration
conducted on a 0.4 mmol scale; cyclization reaction went to completion overnight.
