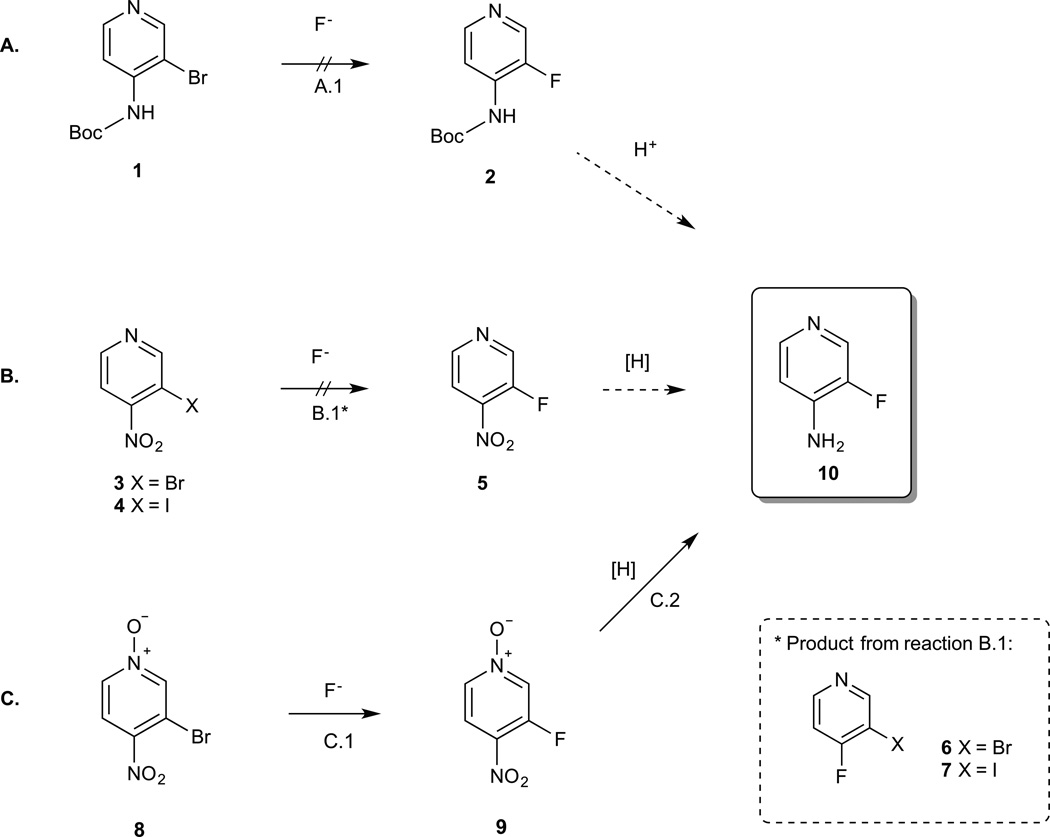
Scheme 1. Possible fluorination strategies for 3-fluoro-4-aminopyridine.
A. Nucleophilic aromatic substitution of 3-bromo-4-(boc-amino)pyridine (1) followed by acid deprotection. Fluorination did not proceed under the experimental conditions (A.1: 1 eq TBAF, 125 C,DMSO, 16 h). B. Nucleophilic aromatic substitution of 3-bromo-4-nitropyridine (3) or 3-iodo-4-nitropyridine (4) followed by reduction. Under the experimental conditions (B.1: 0.5 eq. TBAF, 25 C, DMSO, 15 min) the product formed was 3-bromo-4-fluoropyridine (6) or 3-iodo-4-fluoropyridine (7) instead of the desired 3-fluoro-4-nitropyridine (5). C. Nucleophilic aromatic substitution of 3-bromo-4-nitropyridine N-oxide (8) followed by reduction. Fluorination produced 3-fluoro-4-nitropyridine N-oxide (9) in 37% yield (C.1: 0.5 eq. TBAF, 25 C, DMSO, 5 min) and hydrogenation of 9 (3 mg of 10% Pd/C, 1 atm H2, MeOH, 25 C, 10 min) produced 3-fluoro-4-aminopyridine (10) quantitatively.