Abstract
Malaria vaccine development has been hampered by the limited availability of antigens identified through conventional discovery approaches, and improvements are needed to enhance the efficacy of the leading vaccine candidate RTS,S that targets the circumsporozoite protein (CSP) of the infective sporozoite. Here we report a transcriptome-based approach to identify novel pre-erythrocytic vaccine antigens that could potentially be used in combination with CSP. We hypothesized that stage-specific upregulated genes would enrich for protective vaccine targets, and used tiling microarray to identify P. falciparum genes transcribed at higher levels during liver stage versus sporozoite or blood stages of development. We prepared DNA vaccines for 21 genes using the predicted orthologues in P. yoelii and P. berghei and tested their efficacy using different delivery methods against pre-erythrocytic malaria in rodent models. In our primary screen using P. yoelii in BALB/c mice, we found that 16 antigens significantly reduced liver stage parasite burden. In our confirmatory screen using P. berghei in C57Bl/6 mice, we confirmed 6 antigens that were protective in both models. Two antigens, when combined with CSP, provided significantly greater protection than CSP alone in both models. Based on the observations reported here, transcriptional patterns of Plasmodium genes can be useful in identifying novel pre-erythrocytic antigens that induce protective immunity alone or in combination with CSP.
Introduction
Malaria is estimated to have caused 854,568 deaths worldwide in 2013 [1], and existing tools for prevention and treatment are losing their efficacy. Nearly five decades after the first demonstration of sterile protection induced by multiple exposures to radiation-attenuated sporozoites (RAS) in mice [2] and humans [3], the world still awaits a licensed and effective malaria vaccine that blocks infection. Manufacturing of RAS for mass-immunization is still in development, while the leading subunit vaccine RTS,S shows 37% efficacy in African children and no significant efficacy in infants against severe malaria [4–6].
RTS,S is based on the Plasmodium falciparum (Pf) circumsporozoite protein (CSP), the major surface antigen of the sporozoite stage (SS) (6). RTS,S-induced protection from clinical malaria is thought to be mediated by antibodies against the central repeat region together with CD4+ T cells [7–9]. Protection against infection correlates most strongly with antibodies induced by RTS,S [10,11], as does sterile immunity induced by the RAS clinical product called PfSPZ Vaccine® that has been tested in humans [12]. Protection induced by RAS in mice [13–15] and in non-human primates [16] depends on CD8+ T cells, suggesting that intracellular (intra-hepatocytic) parasite antigens are targets of immunity.
CSP is the immunodominant antigen in RAS vaccines. Although RAS efficacy is significantly reduced in transgenic mice that are tolerized to CSP, increasing the number of doses induces immunity that completely blocks infection in these mice [17–19]. Thus other antigens are likely involved in RAS-induced protection. Human seroreactivity studies similarly suggest that immunity to other antigens expressed by pre-erythrocytic parasites (SS and liver stage (LS)) might enhance CSP-induced protection [20]. No antigens have been shown to enhance the efficacy of RTS,S when used in combination in humans. The thrombospondin-related anonymous protein (TRAP) has been incorporated in a multiantigen virus-vectored platform for heterologous prime boost immunizations (ChAd63-MVA ME-TRAP) which induce partial protection against Pf infection [21,22]. Interestingly, RTS,S showed no protective efficacy when combined with recombinant TRAP protein in an AS02 adjuvant formulation [23], emphasizing that some protective immunogens may not be useful in combination with CSP-based vaccines.
Improvements in pre-erythrocytic vaccines have been stymied by our limited knowledge of Pf LS antigens. In this paper, we report the identification of protective pre-erythrocytic antigens by transcriptome profiling of Pf LS parasites. Candidate antigens were selected from among genes that are over-transcribed in LS compared to either SS or blood stage (BS) parasites. Several of these antigens conferred partial protection against infectious sporozoite challenge in two mouse models: P. berghei (Pb) in C57Bl/6 (B6) and P. yoelii (Py) in BALB/c. When combined with CSP, the sporozoite and liver stage asparagine-rich protein (SLARP) [24], also known as SAP1 [25], and liver-specific protein 1 (LISP1) [26,27] reduced LS burden beyond the reduction achieved with CSP alone in both mouse models. Protection induced by these antigens was mediated by diverse immune mediators including CD8+ T cells. We conclude that Pf proteins whose genes are over-transcribed during LS development include many protective pre-erythrocytic antigens that might contribute to the development of an effective anti-infection malaria vaccine, including antigens that can be combined with CSP.
Materials and Methods
Parasite maintenance
Pf (NF54 strain) was transmitted to Anopheles stephensi mosquitoes by standard membrane feeding with infected human blood. Pb (ANKA strain) and Py (17XNL strain) sporozoites were prepared by cyclical transmission in BALB/c mice and Anopheles stephensi mosquitoes. Sporozoites were dissected from the salivary glands of mosquitoes on day 21 (Pb), day 15 (Py) or day 18 (Pf) after an infective blood meal, as described previously [28,29]. For challenge studies, sporozoites were counted, adjusted to a given concentration in RPMI 1640 (Life Technologies, Grand Island, NY) with 0.1% normal mouse serum, and used immediately after dissection to ensure maximal infectivity. Pf sporozoites were suspended in DMEM/F-12 medium supplemented with 10% FBS, 100 units/ml penicillin and 100 μg/ml streptomycin, and used for axenic culture as described previously [30], or used for in vitro infection of HC-04 cells [31].
For microarray analysis, Pf parasites were isolated from the blood of Tanzanian children aged between 1 and 4 years [32]. These are the children enrolled in a cohort known as Mother-Offspring Malaria Studies (MOMS), after written informed consent from their mother. Laboratory Pf (strain NF54) was cultured in vitro in erythrocytes isolated from whole blood as described earlier [33].
Tiling microarray studies
Custom Pf arrays were used to measure the expression profile of genes at different developmental stages, including SS, LS using 24h and 48h axenically cultured parasites, and BS parasite samples collected from Tanzanian children with mild malaria. The array contains 386225 probes, varying in length from 50 to 75 bp (GEO Accession ID: GSE56959), tiling the entire Pf genome. RNA samples were sent to Roche-Nimblegen (Roche Nimblegen, Inc., USA) for hybridization and quantification of raw probe intensities. Raw probe intensities were processed with in-house software (R package ‘DuffyMA’). Slides were normalized using Robust Multichip Averaging (RMA) method described in earlier publications [34–36]. Transcript expression was calculated as the geometric mean of all exonic probe intensities for each gene.
Quantitative real-time PCR (qPCR) verification of Pf gene transcription
Total RNA was extracted from the following NF54 strain Pf samples: salivary gland sporozoites, infected HC-04 cell samples (24, 48, and 72 h after infection), mixed BS, and uninfected HC-04 cells (negative control). cDNA was prepared from 15 μg of total RNA using the Superscript II RT kit (Invitrogen, Carlsbad, MD) according to manufacturer’s instructions, and qPCR was performed as described earlier [37].
qPCR primer sets were designed using the online program Primer3 [38]. Two hexokinase (PF3D7_0624000) primer sets were used as endogenous controls for data normalization. All primer sequences are available in S1 Table. Data were analyzed according to the relative-standard curve method; standard curves were composed of six 10-fold dilutions of 3D7 genomic DNA.
Construction of DNA vaccines
The genes listed in Table 1 were amplified by PCR from Py (17XNL) and Pb (ANKA) genomic DNA or cDNA to produce inserts for ligation-independent cloning. Inserts were then cloned into the mammalian expression plasmid VR1020 (obtained under MTA from Vical Corporation) that was modified to add ligation-independent cloning sites (forward: 5’-CG- CCCAGCGGCACCGGCATG-3’; Reverse: 5’-CACGCACGCGAGCGGGCTTA-3’) using two rounds of site-directed mutagenesis (Stratagene QuikChange II Kit). Because, SLARP, LISP1 and orthologues of PF3D7_0103400 and PF3D7_0304300 are large proteins, multiple fragments were designed, and attempts were made to clone each of them. Of those attempted, we were able to generate 2 out of 6 attempted for PySLARP, 1 of 4 for PbSLARP, 3 of 3 for PyLISP1, 2 of 5 for PbLISP1, 1 of 2 each for PbPF3D7_0304300 and PyPF3D7_0304300, 1 of 3 for PyPF3D7_0103400, and neither of the 2 attempted for PbPF3D7_0103400. Primer sequences for successfully cloned genes are included in S2 Table.
Table 1. Pre-erythrocytic antigens selected for vaccine evaluation.
| Pf Name | Py ortholog | Pb ortholog | Protein Function | A.A. boundaries in vaccine | |
|---|---|---|---|---|---|
| Py | Pb | ||||
| PF3D7_0304600 (CSP) | PY03168 | PBANKA_040320 | Circumsporozoite (CS) protein (CSP) | 35–380 | 21–320 |
| PF3D7_0730200a | PY03000 | PBANKA_021430 | Adapter-related protein | 6–891 | 5–892 |
| PF3D7_1323000a | PY01586 | PBANKA_133820 | Beta-hydroxyacyl-ACP dehydratase (FabZ) | 8–231 | 1–229 |
| PF3D7_1411500a | PY00162 | PBANKA_103100 | Conserved Plasmodium protein, unknown function | 3–980 | 302–942 |
| PF3D7_1418100 (LISP1)a | PY04499 | PBANKA_102460 | Liver specific protein 1, putative (LISP1) | 25–607, 600–1178, and 1172–1732 | 28–601 and 2579–3254 |
| PF3D7_1207400a | PY04162 | PBANKA_060590 | Conserved Plasmodium protein, unknown function | 1–387 | 153–348 |
| PF3D7_1241500a | PY01495 | PBANKA_145490 | Conserved Plasmodium protein, unknown function | 1–467 | 1–298 |
| PF3D7_1308500b | PY04905 | PBANKA_140700 | Conserved Plasmodium protein, unknown function | 14–453 | 319–456 |
| PF3D7_0727200b | PY02096 | PBANKA_021130 | Cysteine desulfurase, putative (NFS) | 3–559 | 1–541 |
| PF3D7_0818900b | PY06158 | PBANKA_071190 | Heat shock 70 KDa protein, (HSP70) | 1–682 | 1–693 |
| PF3D7_1111200b | PY02705 | PBANKA_093640 | Conserved Plasmodium protein, unknown function | 1–360 | 122–553 |
| PF3D7_1122200b | PY05603 | PBANKA_092610 | Cupin-like protein, putative | 63–350 | 63–447 |
| PF3D7_1134000b | PY06981 | PBANKA_091440 | Heat shock protein 70 (Hsp70-3) | 1–663 | 250–553 |
| PF3D7_1147000 (SLARP)b | PY03269 | PBANKA_090210 | Sporozoite and liver asparagine-rich protein (SLARP) | 1356–1989 and 2554–2599 | 2877–3218 |
| PF3D7_1302200b | PY03011 | PBANKA_140080 | Early transcribed membrane protein 13 (ETRAMP13) | 21–215 | 1–209 |
| PF3D7_1434400b | PY03139 | PBANKA_101040 | Conserved Plasmodium membrane protein, unknown function | 1–264 | 29–261 |
| PF3D7_1456100b | PY00669 | PBANKA_131980 | Serine hydroxymethyltransferase, putative (SHMT) | 1–484 | 5–484 |
| PF3D7_0103400b | PY03811 | PBANKA_020970 | Zinc-carboxypeptidase, putative | 623–1203 and 1214–1563 | N/A |
| PF3D7_0304300b | PY03529 | PBANKA_040290 | Conserved Plasmodium protein, unknown function | 15–754 | 1–612 |
| PF3D7_0405500b | PY01668 | PBANKA_100320 | Conserved Plasmodium protein, unknown function | 1–198 | 1–199 |
| PF3D7_0506200b | PY00712 | PBANKA_110580 | Transcription initiation factor TFiid, TATA-binding protein (TBP) | 1–277 | 35–281 |
| PF3D7_1026400c | PY07496 | PBANKA_051060 | Cell division cycle protein 20 homolog, putative (CDC20) | 291–552 | 1–538 |
aProtective in both models
bProtective in one model
cProtective in neither model
CSP as a positive control.
One Shot TOP10 Chemically Competent E. coli bacteria (Invitrogen) were transformed with plasmids. Positive clones were selected by DNA sequencing. Sequence-verified clones were used for plasmid DNA preparation for immunization. Plasmid DNA was purified using Geneelute Endotoxin Free Maxiprep kits (Sigma, St. Louis, MO) or Plasmid Endo-Free purification kits (Maxi or Giga, Qiagen, Netherlands) according to manufacturer’s instructions. Plasmids were resuspended at 1 μg/μL in 1X Tris-EDTA buffer (Ambion, Waltham, MA) for Gene Gun (GG) cartridge preparation, at 2 μg/μl in sterile 1X phosphate-buffered saline for intramuscular (IM) injection, and at 0.5 μg/μl in 0.9% NaCl for electroporation (EP) delivery. GG cartridges were prepared as described earlier [39].
Ethics statement
All procedures were reviewed and approved by Walter Reed Army Institute of Research/Naval Medical Research Center Institutional Animal Care and Use Committee (protocols 12-MVD-31 and 15-MVD-30), National Institutes of Health Institutional Animal Care and Use Committee (protocol LMIV 1E) and Seattle Biomedical Research Center Institutional Animal Care and Use Committee (protocols PD-02 and AO-02) and were performed in a facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in compliance with the Animal Welfare Act and in accordance with the principles set forth in the “Guide for the Care and Use of Laboratory Animals”, Institute of Laboratory Animals Resources, National Research Council, National Academy Press, 1996. For Pf culture, fresh human blood was provided by employee volunteers from SBRI with informed written consent, under the protocol “SBRI Blood Donor Program”, approved by Western IRB.
Mouse immunizations
Female BALB/c mice, 4–5 weeks old, purchased from Charles River Laboratories. C57Bl/6 (B6) mice and C57Bl/6 × BALB/c F1 mice (CB6F1) (6–8 weeks old) were purchased from The Jackson Laboratories. Mice were maintained under pathogen-free conditions in animal facilities and were fed with autoclaved food ad libitum.
For protection studies, B6 mice were immunized with Pb DNA vaccines, and BALB/c mice were immunized with Py DNA vaccines thrice at 3 week intervals using one of three different routes. Biolistic gold particles coated with plasmid DNA were delivered into shaved mouse abdomens using Helios GG (BioRad, Hercules, CA) at 300 psi of helium gas pressure. Each dose consisted of 2 cartridges at 2.5 μg plasmid DNA/cartridge (total 5 μg plasmid DNA per mouse). For IM injections, 25 μg of plasmid DNA encoding Py or Pb antigens were mixed with 35 μg of GM-CSF plasmid. EP of 10 μg (Pb) or 20 μg (Py) of plasmid DNA into anterior tibialis muscle was done using Intramuscular TriGrid Delivery System (Ichor Medical Systems, San Diego, CA) according to manufacturer’s recommendations. Two weeks after the last immunization, B6 mice were challenged with 10,000 Pb sporozoites and BALB/c mice were challenged with 20,000 Py sporozoites intravenously. Forty to 42 hours after the challenge, livers were harvested for LS burden estimation. For CSP combination immunization studies, mice were immunized three times, 3 weeks apart, by GG for Pb and by EP for Py. Doses were 2.5 μg plasmid DNA each of individual antigen and CSP for GG, and 10 μg plasmid DNA each for EP. Control mice were immunized with 2.5 μg plasmid DNA of both CSP and empty plasmid vector (EV) control for GG, and with 10 μg plasmid DNA of both for EP. LS burden was estimated after sporozoite challenge by qPCR as described below.
Evaluation of vaccine efficacy
Total RNA was extracted from liver samples using RNeasy mini kit (Qiagen) (Py) or Trizol (Pb) according to manufacturer’s instructions. cDNA was synthesized using Superscript III RT kit (Invitrogen) (Py) or High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) (Pb). Gene expression analysis for Py was carried out with 1:40 dilution of cDNA. The qPCR reactions included 1X ABI PowerSYBR (Applied Biosystems) and 0.25 μM of either Py 18S rRNA primers (5’-GGGGATTGGTTTTGACGTTTT-3’ and 5’-AAGCATTAAATAAAGCGAATA-3’) or mouse β-actin primers (5’-GGCTGTATTCCCCTCCAT-3’ and 5’-CCAGTTGGTAACAATGCAAT-3’) in a 20 μl volume. qPCR reactions were run on ABI 7500 machine, using the 9600 emulation protocol with annealing/extension step modified to 57.5°C.
Pb reaction samples contained the following reagents in 25 μl volume: 12.5 μl of SYBR Green PCR Master Mix (Applied Biosystems), 0.1 μM of either Pb 18S rRNA primers (5’-AAGCATTAAATAAAGCGAATACATCCTTAC-3’ and 5’-GGAGATTGGTTTTGACGTTTATGTG-3’) or mouse β-actin gene (5’-GGCTGTATTCCCCTCCAT-3’ and 5’-CCAGTTGGTAACAATGCAAT-3’) and 2 μl of 1:10 dilution of cDNA sample. The reaction was run on 7500 Fast qPCR System (Applied Biosystems) using the following conditions: 15 min at 95°C, 40 cycles with 95°C for 20 s; 60°C for 30 s and 72°C for 50 s.
cDNA standards were prepared as 10-fold serial dilutions of purified PCR product for both 18S rRNA and β-actin from 108 to 105 copies. 18S rRNA was used as an internal control and each reaction was set up in triplicate. Liver of naive mouse served as a negative control. Parasite load was calculated as ratio of 18S rRNA to host β-actin expression. Protection was defined as a statistically significant reduction of parasite burden in the livers of experimental mice compared to mice immunized with EV and computed as described in statistical methods.
Measurement of CD8 responses to DNA immunization
Two weeks after the final DNA immunization, spleens were harvested from mice and single cell suspensions were prepared from RBC-lysed splenocytes. Cells were stimulated in the presence of brefeldin A with pools of peptides (S3 Table) predicted to have strong binding to H-2Kd/b, Dd/b, Ld based on the Artificial neural network (ANN) method (www.iedb.org) for 16 hours at 37 °C. Cells were then fixed and permeabilized for intracellular IFN-γ, CD90, and CD8. Stained samples were acquired on a BD LSRII and analyzed using FlowJo v9.6 (Tree Star Inc., Ashland, OR).
In vivo depletion of CD8+ T cells
To deplete CD8+ T cells, mice were injected intraperitoneally with 100 μg of purified rat anti-mouse-CD8β antibodies (clone 53–6.8, BD Pharmingen, San Diego, CA) 26-28h before the challenge with Py sporozoites. Efficiency of depletion was confirmed by surface staining of peripheral blood mononuclear cells (PBMC) prior to the challenge, and of splenocytes at 40h after the challenge, using anti-mouse-CD8α, -CD3 and -CD4 antibodies (see “Flow cytometry”).
Flow cytometry
The following anti-mouse antibodies from BD Biosciences were used: FITC-conjugated anti-CD3e (clone 145-2C11), PerCP conjugated anti-CD4 (clone GK1.5), and APC-conjugated anti-CD8α (clone 53–6.7). Liver cells and splenocytes were stained according manufacturer recommendations. The cells were washed with PBS + 2% FBS (Hyclone; GE Life Sciences, Logan, UT) and fixed with 4% formaldehyde in PBS. Live/Dead Fixable Dead Cell Stain Kit for UV excitation (Invitrogen) was used to exclude dead cells. Flow cytometry was performed using LSRII system (BD Biosciences, San Jose, CA) and data were analyzed by FlowJo (v. 9.4.10,) software.
Generation of transgenic parasites
We used a b3D-myc vector for epitope tagging genes of interest [40], to generate the Py myc-tagged transgenic lines of PyPF3D7_0506200, PyPF3D7_1241500, and PyPF3D7_0730200. Genes without their stop codons, together with approximately 1.5 kb sequence upstream from the start codon, were amplified by PCR from Py 17XNL genomic DNA and inserted upstream of the quadruple myc tag in b3D-myc (list of primers given in S4 Table). The plasmids were subsequently linearized with Psr I and integrated into the Py 17XNL genome using standard procedures [41]. This integration strategy created two copies of the target gene, both under the control of the endogenous promoter: the original gene and the myc epitope-tagged gene.
In vitro IFA with myc-tagged Py LS parasites
Immunofluorescence of myc-tagged LS of Py parasite was performed as described earlier [42]. Briefly, HepG2-CD81 cells (5 × 104 Cells/well) were cultured overnight on 8-well chamber slides (LabTek, Rochester, NY) in DMEM medium with 10% FBS at 37°C and 5% CO2. The following day, salivary gland sporozoites dissected from mosquitoes were incubated in RPMI with 20% FBS for 30 min at room temperature. Sporozoites (5 × 104) were added to each well of growing HepG2-CD81 cells and incubated for 48h, with media changed every 24h. Infected cells were washed twice with PBS and fixed with formalin, permeabilized, and blocked with 2% BSA in 0.2% Triton X-100 in PBS for 1h at 37°C. Cells were then stained with mouse anti-myc antibody. Alexa-594 or -488-conjugated anti-mouse IgG was used to detect the recombinant protein, rabbit polyclonal antibody to detect UIS4, and DAPI to stain the nucleus. The slides were mounted using FluoroGuard antifade reagent. Fluorescent images were acquired using Olympus 1 X 70 DeltaVision microscope.
Statistical analysis
Differential gene expression in tiling microarrays was calculated as the ratio of intensity levels between stages, with significance determined by the two sample t-test of the log intensities of all exonic probes. Gene rank percentiles were calculated as a uniform distribution from 0 to 100 based on their ordinal position among all 5536 genes for microarrays, or for the 131 genes assayed for qPCR studies, such that the gene with highest transcription in each sample has a rank percentile of 100.
For meta-analysis of all LS burden studies parasite load was converted to percent reduction for all mice from each study by the equation: , using only the EV mice from the corresponding study. This yields a PctReduction value for all experimental mice and for all control mice, to facilitate combining studies and measuring the statistical significance of each antigen. All groups were compared by Kruskal-Wallis test to determine if differences existed, followed by the non-parametric, 2-sample, 1-sided Mann-Whitney test (R command ‘wilcox.test’) to generate a P-value for individual antigens. The antigen was called protective when the P-value ≤ 0.05 in a comparison of antigen-immunized mice to EV-immunized mice within the same studies. Assays in which none of the antigens including positive control CSP conferred protection were considered uninterpretable and excluded from further analysis. This same strategy was used for both the individual antigen immunization studies and for the CSP combination immunization studies, with the only difference being that the comparator group for the latter studies received EV + CSP combination vaccine.
Results
Identification of candidate pre-erythrocytic vaccine antigens
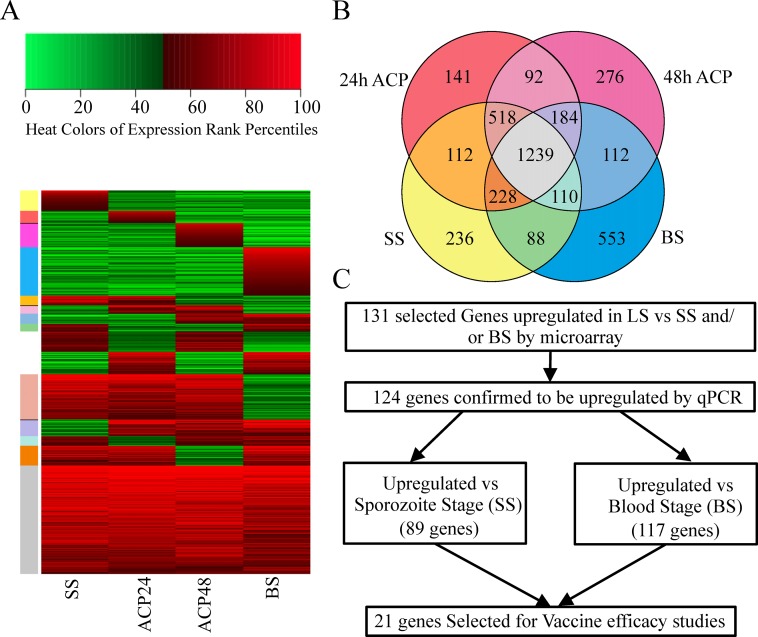
We cultured NF54 strain Pf sporozoites for 24 and 48 hours under axenic conditions [30] to generate material suitable for transcriptional profiling of early Pf LS. LS transcriptome data were generated by tiling microarray (Accession No: GSE56959), and we compared the transcriptomes of 24h and 48h LS with those of NF54 strain SS and of BS collected directly from children with mild malaria (Fig 1A and 1B). We identified genes with higher rank percentile transcription at any time point during LS, as compared to either SS or BS, anticipating that this would enrich for genes encoding LS antigens. Using qPCR on parasites infecting HC-04 hepatocytes, we verified that 124 genes among a total of 131 tested (primer sequences listed in S1 Table), were upregulated in LS compared to NF54 strain SS and/or NF54 strain BS transcription. Eighty-nine genes were upregulated during LS compared to SS and 117 were upregulated compared to BS (Fig 1C).
Fig 1. Gene expression profile of Pf genes and selection algorithm used for antigen selection.
(A) Heat map of gene expression by tiling microarray. Red color represents the genes expressing above the 50th percentile, while green color represents genes expressing below the 50th. The heat map shows the expression profile of genes across SS, 24h and 48h axenically cultured LS, and BS parasites. (B) Venn diagram shows stage-specific expression of genes by tiling microarray. Color coding of Venn regions matches the color bar provided for the heat map in Panel A. (C) Selection of genes for vaccine evaluation in two rodent models. One hundred and thirty-one genes were selected from among the upregulated genes identified by tiling-microarray, and 124 were confirmed by qPCR to be transcribed at higher levels in LS versus SS and/or BS parasite samples. Twenty-one of these 124 genes were selected for further evaluation as vaccine candidates.
Evaluation of vaccine efficacy in Py and Pb models
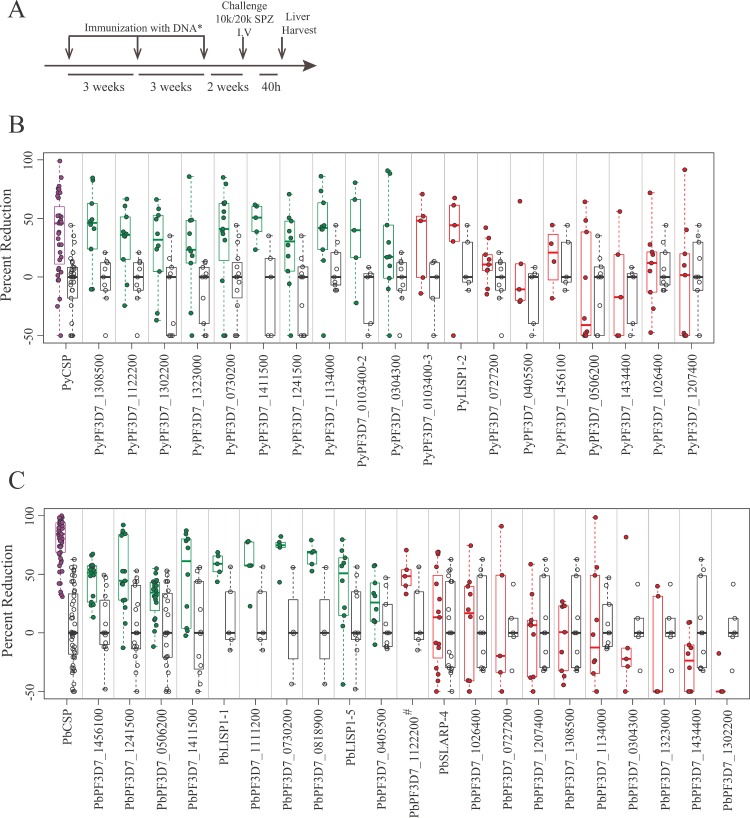
Twenty-one genes upregulated in Pf LS parasites and with predicted orthologues in Py and Pb were selected to conduct immunization/challenge studies in two rodent models used for initial and confirmatory screening (Py in BALB/c and Pb in B6 mice, respectively) (Fig 1C and Table 1). The selected candidates included genes with or without predicted export sequences (e.g., canonical eukaryotic signal peptide (SP) or transmembrane (TM) domains, or parasite-specific PEXEL/VTS motifs [43,44]), and with varying patterns of transcription across SS, LS and BS parasites (Table 2). CSP DNA vaccine was used as a positive control and benchmark of partial protection [45], and EV served as a negative control. Mice were immunized 3 times with DNA constructs, and then challenged with homologous sporozoite species (Fig 2A); protection conferred by each DNA vaccine was measured as reduction of LS parasite burden (18S rRNA level quantified by qPCR) compared to EV control tested in the same immunization studies (Fig 2B and 2C). Using GG immunization, 10/21 novel antigens significantly (P<0.05) reduced LS burden compared to the EV immunized group in the Py model (Fig 2B and S5 Table) as did 9/21 in the Pb model (Fig 2C and S5 Table), with 3 antigens (orthologues of PF3D7_0730200, PF3D7_1241500 and PF3D7_1411500) demonstrating protection in both models.
Table 2. Protein features and transcriptional profiles of selected candidate genes.
| Pf Gene Name | Protein Feature | Upregulated transcription in LS vs | ||||
|---|---|---|---|---|---|---|
| Protein Length | TM | SP | PEXEL/VTS | BS | SS | |
| PF3D7_0304600 (CSP) | 397 | 1 | 1 | 2 | Yes | No |
| PF3D7_0730200a | 858 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_1323000a | 230 | 1 | 1 | 0 | Yes | Yes |
| PF3D7_1411500a | 947 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_1418100 (LISP1)a | 3597 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_1207400a | 395 | 0 | 0 | 0 | Yes | No |
| PF3D7_1241500a | 420 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_1308500b | 359 | 0 | 0 | 0 | Yes | No |
| PF3D7_0727200b | 553 | 0 | 0 | 1 | Yes | No |
| PF3D7_0818900b | 677 | 0 | 0 | 1 | Yes | Yes |
| PF3D7_1111200b | 594 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_1122200b | 446 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_1134000b | 663 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_1147000 (SLARP)b | 2940 | 0 | 0 | 0 | Yes | No |
| PF3D7_1302200b | 229 | 2 | 1 | 0 | Yes | No |
| PF3D7_1434400b | 285 | 1 | 0 | 0 | Yes | No |
| PF3D7_1456100b | 462 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_0103400b | 1620 | 0 | 0 | 0 | Yes | No |
| PF3D7_0304300b | 1429 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_0405500b | 224 | 0 | 0 | 1 | Yes | Yes |
| PF3D7_0506200b | 327 | 0 | 0 | 0 | Yes | Yes |
| PF3D7_1026400c | 603 | 0 | 0 | 0 | Yes | No |
aProtective in both models
bProtective in one model
cProtective in neither model
CSP as a positive control.
Fig 2. GG DNA immunization and reduction of LS parasite burden post-sporozoite challenge.
(A) Experimental design for the immunization and challenge studies. Mice were immunized 3 times at 3 week intervals with VR1020 plasmid DNA carrying the Pb or Py antigen. Two weeks after the last boost mice were challenged with 10,000 Pb or 20,000 Py sporozoites intravenously and livers were harvested 40h post-challenge. *DNA dose is 5 μg (GG), 25 μg + 35 μg GM-CSF DNA (IM) or 20 μg (EP). (B) Meta-analyses of 7 independent immunization experiments and resulting LS parasite burden reduction in Py in BALB/c model by GG immunizations. (C) Meta-analyses of 10 independent immunization experiments and resulting LS parasite burden reduction in Pb in C57Bl/6 model induced by GG immunizations. Each circle represents one mouse. Green color indicates significant difference as compared to EV immunized groups tested in the same immunization studies (p<0.05). Red color indicates p>0.05 and therefore no significant difference in LS parasite burden reduction as compared to EV immunized group. Purple color indicates LS parasite burden reduction by CSP (positive control). A complete statistical analysis is provided in S5 Table.
Protective efficacy of antigens can vary depending on the route of immunization [45]. Therefore, in addition to GG immunization, we tested many of the Py and Pb immunogens using EP, and in addition IM injection for many Py immunogens. Most (6/10) DNA vaccines that protected by GG immunization for Py also conferred functional immunity when delivered by IM and/or EP routes; 4/9 Pb immunogens that conferred protection by GG also conferred protection by EP administration (S1 Fig). In some cases, protection was only seen with one route of administration: for example, Py antigens PyPF3D7_1026400and PyPF3D7_1207400 only achieved significant protection by either EP or IM delivery respectively. Notably, the sample size was not uniform across antigens, in part because some experiments were uninterpretable; for this reason the power to detect differences varied across antigens, and therefore some differences of relatively smaller magnitude were significant while those of greater magnitude did not achieve significance. GG immunization has been thought to confer greater protective efficacy [46] in comparison to the IM route, but IM immunization may achieve superior results in a subset of antigens [45]. For unclear reasons, IM administration yielded poor protective activity in the Pb model and was hence discontinued after testing only a minority of the antigens (data not shown). In total, 6 antigens (orthologues of PF3D7_1411500, PF3D7_0730200, PF3D7_1241500, PF3D7_1323000, PF3D7_1207400 and LISP1) significantly reduced LS burden after sporozoite challenge in both Py and Pb mouse models of malaria, by at least one route of administration (Fig 2, S1 Fig and S5 Table). The observation that most antigens conferred protection with more than one delivery platform, and also that they conferred protection in more than one mouse model, suggest that these are robust targets for further vaccine development.
Protection induced by novel LS antigens is mediated by diverse mechanisms
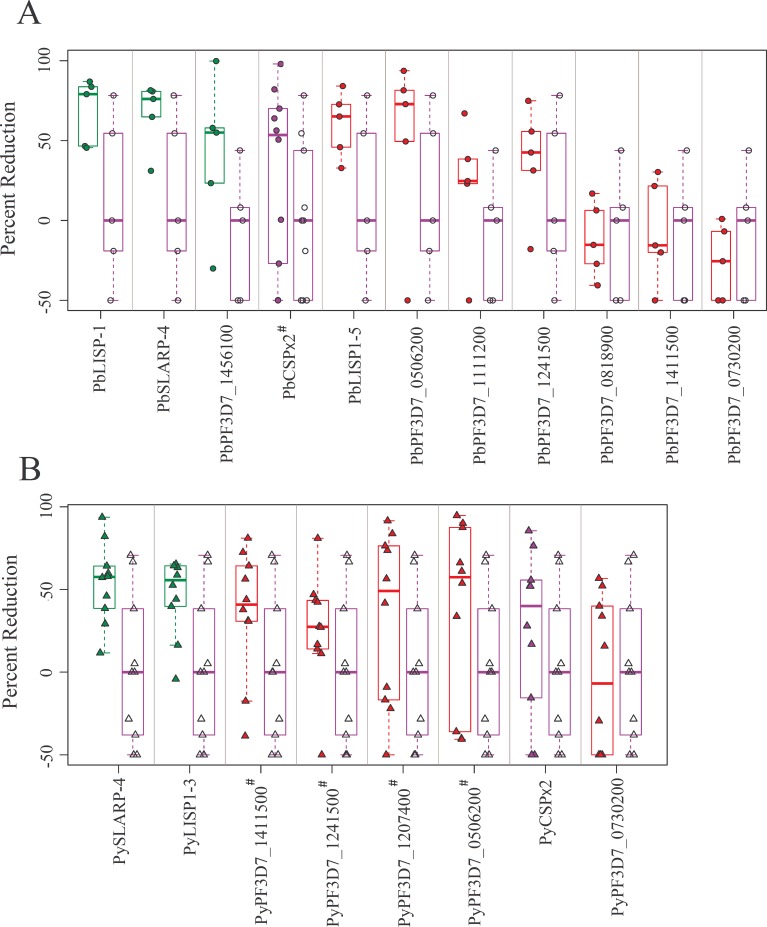
Previous studies in rodents demonstrated that protective immunity induced by vaccination with RAS is mediated by multi-factorial mechanisms including neutralizing antibodies that block sporozoite invasion and effector CD8+ T cells that eliminate infected hepatocytes. To investigate if CD8+ T cells consistently contribute to protection induced by novel LS antigens, we confirmed that 4 antigens conferred protection in CB6F1 hybrid mice, an additional mouse model which we surmised might yield more diverse immune responses owing to its more diverse MHC haplotypes. Three of these antigens (PyPF3D7_1456100, PySLARP, PyPF3D7_1207400) elicited IFN-γ responses from CD8+ T cells, while PyPF3D7_0506200 apparently did not (S2A Fig), based on stimulation with a pool of peptides predicted to be CD8+ T cell epitopes for H-2Kd/b, Dd/b, Ld by the Artificial neural network (ANN) method (www.iedb.org). CB6F1 mice immunized by GG with these Py DNA antigens, as well as benchmark antigen PyCSP, received anti-CD8β antibodies to deplete CD8+ T cells 24 hours before the challenge with Py sporozoites (S2B Fig). We had previously determined that a single i.p. injection with 100μg of anti-CD8 monoclonal antibodies one day prior to challenge eliminated >80% of CD8+ T cells in peripheral blood and spleen (S3 Fig) and that administration of RatIg control antibody had no effect on CD8+ T cells (data not shown). CD8 depletion completely abrogated protection induced by novel LS Ags PyPF3D7_1456100 and PySLARP in CB6F1 mice (S2B Fig). Protection induced by PyPF3D7_1207400 was partially mediated by CD8+ T cells–reduction of LS parasite burden dropped from 31% to only 13% after depletion. Depletion of CD8+ T cells did not cause any loss of protection in mice immunized with PyPF3D7_0506200, the antigen for which we could not detect CD8+ T cell responses after DNA vaccination. Notably, CSP induced protection did not require CD8+ T cells in this model, despite the fact that the CSP immunogen elicited peptide-specific CD8+ T cell responses. Our data suggest that protection induced by novel pre-erythrocytic antigens can be mediated by diverse mechanisms that vary by antigen, some of which include induction of effector CD8+ T cells.
Efficacy of combination vaccines with CSP and novel antigens
The leading human malaria vaccine is based on the antigen CSP. CSP reduces LS burden and induces partial protection when delivered as a DNA vaccine in the Pb and Py models [45,47]. We assessed whether the addition of our novel antigens might provide greater protection than that achieved with CSP alone.
Several antigens that demonstrated partial protection as individual immunogens in one or both of our animal models (7 Py and 9 Pb antigens) were selected for combination vaccine studies. Among these, PySLARP-4 and PyLISP1-3 were protective by IM delivery, but protection by GG could not be interpreted due to QC criteria. Animals that received the combination of CSP and a novel antigen were compared to those immunized with CSP plus EV tested in the same immunization studies. None of the selected antigens interfered with protection induced by CSP alone, and in general, LS burden trended lower in mice that received combination vaccines. Two Py antigens (PyLISP1 and PySLARP) and 3 Pb antigens (PbLISP1, PbSLARP and PbPF3D7_1456100) conferred significantly greater protection than that seen with CSP plus EV (Fig 3 and S6 Table).
Fig 3. Novel antigens combined with CSP provide greater protection than CSP alone.
(A) Each C57Bl/6 mouse (circles) was immunized with a combination of PyCSP DNA (2.5 μg) with novel antigen DNA (2.5 μg) by GG, using the schedule described in Fig 2. (B) Each BALB/c mouse (triangles) was immunized with a combination of PbCSP DNA (10 μg) with novel antigen DNA (10 μg) by EP, using the same schedule used for the GG immunizations. Data were compared to the negative control group immunized with a combination of CSP and EV tested in the same immunization study. Significant reduction in LS parasite burden was determined by Kruskal-Wallis test followed by Mann-Whitney test and p<0.05 was considered as significant. Green box indicates p<0.05 and red box indicates p>0.05. A complete statistical analysis is provided in S6 Table.
Expression and localization of protective antigens
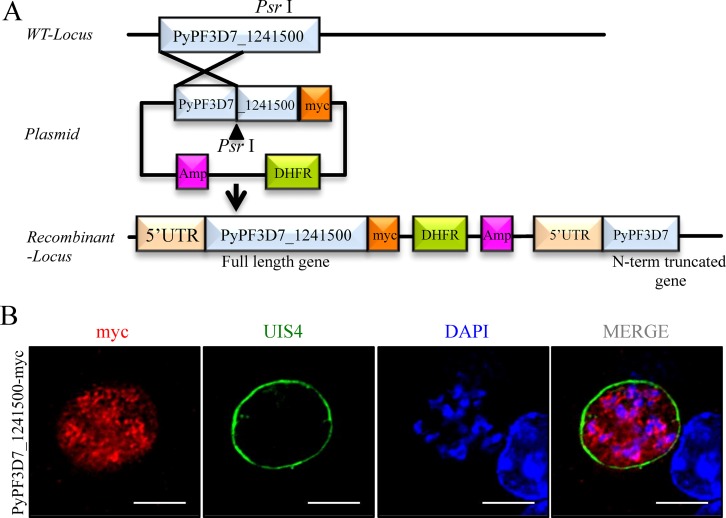
We hypothesized that proteins that were secreted into the host cell cytosol or localized on parasitophorous vacuole membrane (PVM) might be over-represented among our protective antigens, since these proteins should be more accessible for processing and presentation to T cells. Based on in silico analyses, we did not find that antigens protective in both Py and Pb models were more likely to have putative signal peptides, transmembrane domains, or export motifs (PEXEL/VTS) (Table 2). Two proteins (LISP1 and SLARP) were previously reported to be expressed by LS parasites, and these respectively localize to PVM [26,27] and to early LS peri-nuclear region [24]. We studied protein expression of several of our novel vaccine candidates by myc epitope-tagging and immunofluorescence assays (Fig 4A). Myc-tagged PyPF3D7_1241500 was detected in 24h LS and localized to the parasite peri-nuclear area but was not exported (Fig 4B). PyPF3D7_0730200 also appeared in 24h LS and localized in parasite cytoplasm (S4A Fig), while PyPF3D7_0506200 localized to the parasite nucleus (S4B Fig). In summary, the novel candidate vaccine antigens are expressed as LS proteins, but most do not localize to PVM or host cell cytosol.
Fig 4. Expression of novel antigens by Py LS parasites.
(A) Strategy for generation of myc-tagged PyPF3D7_1241500. (B) Immunofluorescence assay using Py17XNL grown 24h in HepG2-CD81 cells, showing expression of PyPF3D7_1241500 protein detected by Alexa-594 conjugated anti-myc antibody (red). UIS4 (green) was used as a PVM marker and DAPI to identify nuclei. Scale bar represents 10 μm.
Additionally, we examined transcriptional profiles of genes in Pf for an association with protection in the mouse models. Among the antigens shown to be protective in both Pb and Py models as single immunogens, 5/6 were transcribed at higher levels in LS versus both SS and BS; only 8 of the other 15 antigens were similarly upregulated versus both SS and BS (Table 2). When analyzing the trajectory of transcription over the first 72 hours of Pf LS development in HC-04 cells, the group of antigens that conferred partial protection in both models showed an average increase (linear regression slope = 0.41/hr, p = 0.051) in their transcription, while antigens that failed to protect in one or both models showed a decline in their transcription (linear regression slope = -0.07/hr, p = 0.54). The linear regression trend, in terms of gene transcription, between the two groups is significantly different (p = 0.04) (S5 Fig).
Discussion
Candidate pre-erythrocytic vaccine antigens have previously been selected by screening with sera or T cells from protected or malaria-exposed individuals, but these have demonstrated only modest or no protective efficacy in human clinical trials [48–52]. We have used a transcriptomic approach to identify novel pre-erythrocytic malaria antigens. Although the model of axenically cultured parasites does not fully mimic the development of intrahepatocytic parasites, the transcriptomes of these parasites appeared similar: 124/131 genes upregulated in axenic culture were also upregulated in parasitized HC-04 hepatic cells. We selected 21 genes that are transcribed at higher levels during LS compared to SS and/or BS parasites, and found that several conferred functional immunity that reduced LS burden in both the Py model used for initial screening as well as the Pb model used for confirmation. Two antigens conferred greater protection when used in combination with CSP versus CSP alone. The antigens that conferred protection in both models are expressed as proteins during Pf LS development and most are transcribed at higher levels in LS versus both SS and BS parasites (Table 2 and S5 Fig). These characteristics provide a rational basis in the future to identify additional candidate antigens for pre-erythrocytic vaccines, while this first generation of novel candidate antigens moves forward for further assessment as vaccines against human malaria.
As a group, the antigens that conferred protection in both murine models were encoded by genes that increase in transcription as early LS development proceeds. Interestingly, the genes that protected only in a single model, did not have this feature, and on average appeared to decrease in transcription (S5 Fig). This feature also distinguishes our protective antigens from CSP, which rapidly decreases in transcription after sporozoite invasion of hepatocytes [53]. The combination of CSP with antigens that are increasing in expression during LS development may enhance immune responses against a wider window of epitopes, which could explain why these combinations were superior to CSP alone.
We used DNA vaccination of mice to down select pre-erythrocytic genes that merit further investigation as candidate human vaccine antigens [45,54]. Mouse malaria models have been used extensively for assessment of candidate malaria vaccines. DNA vaccination of BALB/c mice with the Pb and Py orthologues of CSP confers partial protection against pre-erythrocytic malaria [45,47], providing a benchmark for the level of efficacy seen with new antigens. On average, CSP conferred the highest level of protection in both the Py–BALB/c model and the Pb–B6 model, consistent with evidence that CSP is the immunodominant antigen in response to whole sporozoite vaccines [18]. However, vaccination with several of our novel antigens also significantly reduced the LS parasite burden after sporozoite challenge, and in some cases their efficacy was similar to that observed with CSP (S5 Table). Ideally, these antigens could be developed and tested as individual immunogens in humans, and compared directly to CSP-based vaccines to assess their relative efficacy against experimental infection of humans.
Our primary goal in this work has been to identify antigens that could be combined with CSP in a more efficacious vaccine. CSP-based DNA vaccines are partially protective in these mouse models, and thus well-suited for this assessment. In general, LS parasite burden was lower in animals that received the combination of our novel antigens with CSP, compared to CSP combined with EV. Additional studies are warranted with all the antigens to confirm the significance of these differences. Immunization with LISP1 [26,27] or SLARP [24,25] in combination with CSP afforded greater protection than CSP alone in both animal models, lending strong support for their further development in a combination vaccine. Notably, PbSLARP and PyLISP1 antigens did not perform well when used alone for immunizations. In earlier studies, pre-erythrocytic antigens that conferred a modest degree of protection alone nevertheless achieved a relatively high degree of protection as a combination vaccine in mice [55], similar to our results here.
Two of our candidates were previously shown to be expressed by intrahepatic parasites: SLARP localizes to the cell interior of LS parasites [24], while LISP1 appears to be associated with the PVM surrounding the developing LS [26,27]. Among the antigens that we examined by myc-tagging, all were confirmed to be expressed as protein by Py LS parasites, but their localization varied. PyPF3D7_1241500 localized to the peri-nuclear area, PyPF3D7_0730200 appeared in parasite cytoplasm (S4A Fig), and PyPF3D7_0506200 localized to the parasite nucleus (S4B Fig). Thus, expression but not localization during LS development is a shared feature of our protective antigens. Although we speculated that protein export might enhance processing and presentation to T cells, we could not conclude that antigens with relevant features, including PEXEL motifs, transmembrane domains and signal sequences, were more likely to confer protection in our limited dataset. Five (PF3D7_1411500, PF3D7_0730200, PF3D7_1241500, PF3D7_1207400, and LISP1) out of 6 antigens that conferred protection in both models lacked any of these predicted features (Table 2).
Little is known about the function of most antigens that demonstrated protective activity in these studies. The nuclear protein SLARP is a transcriptional regulator that controls expression of several known LS genes, such as UIS3, UIS4, p36, and EXP-1 [25,56]. Products of UIS genes often function as components of PVM. Targeted deletion of SLARP leads to fast terminal degradation of many UIS and additional non-UIS transcripts [25,56]. SLARP- mutants are able to traverse hepatocytes, invade host cells and form PVM, but are unable to initiate early LS development. The critical role of SLARP in early LS development is an attractive feature for a vaccine candidate.
LISP1 knockout parasites develop normally through blood and mosquito stages, but demonstrate a severe defect in egress from hepatocytes [26], which raises the possibility that parasite egress may be a novel target for pre-erythrocytic vaccines. We recently showed that the BS schizont protein SEA-1 has a role in parasite egress from erythrocytes, and antibodies to SEA-1 reduce parasite burden and severe malaria risk in mouse models and in human immunoepidemiology studies [57]. Antibodies induced by RTS,S vaccine have been associated with protection from infection in humans [10,11], and the strongest correlate of protection induced by RAS vaccine is antibody activity that inhibits sporozoite invasion of hepatocytes [12]. Therefore novel pre-erythrocytic antigens that confer protection through antibody, at least in part, might be appealing partners to combine with RTS,S.
Evidence supporting the effector function of CD8+ T cells against pre-erythrocytic stage of infection is based on RAS vaccination studies in animals [15,58,59] as well as humans [60]. Therefore, we examined the impact of CD8 depletion on levels of protection after vaccination. We used CB6F1 mice as an additional malaria model, because their genetic diversity relative to their parental strains might permit greater diversity in immune responsiveness. Among four of our LS antigens that conferred significant protection in pilot studies with this model, we found that while some antigens (PyPF3D7_1456100 and PySLARP) induced CD8+ T cells required for protection, others (PyPF3D7_1207400) relied only partially on CD8+ T cells, and in one case (PyPF3D7_0506200), CD8+ T cells did not contribute to protection. The results indicate that the antigens identified by transcriptome profiling of LS parasites can mediate protection through different immune mechanisms that do not always involve CD8+ T cells.
In summary, we used transcriptome profiling and DNA vaccine immunization to identify 6 novel antigens that show efficacy in two murine malaria models by inducing diverse immune responses that reduce LS parasite burden. Two antigens in combination with CSP showed significantly greater efficacy than CSP alone in both models. In general, these antigens had the common features of LS expression, and of increasing transcription during early LS development. The antigens LISP1 and SLARP should be given priority for evaluation in combination with a CSP-based vaccine like RTS,S to prevent malaria infection in humans.
Supporting Information
(A) Meta-analyses of 5 independent immunization experiments and resulting LS parasite burden reduction in Py model with IM immunization. (B) Meta-analyses of 7 independent immunization experiments and resulting LS parasite burden reduction in Py model induced by EP immunizations. (C) Meta-analyses of 4 independent immunization experiments and resulting LS parasite burden reduction in Pb model induced by EP immunizations. Each square or triangle represents one BALB/c or B6 mouse, respectively. Green color indicates significant difference as compared to EV immunized groups tested in the same immunization studies (p<0.05). Red color indicates p>0.05 and therefore no significant difference in LS burden as compared to EV immunized mice. CSP (purple) was used as positive control. EV (black) was used as negative control.
(PDF)
(A) Three of four novel antigens delivered as DNA vaccines induced IFN-γ responses recalled in CD8+ T cells by peptides predicted to be CD8+ T cell epitopes for either H-2b or H-2d mouse haplotypes. Spleens from immunized CB6F1 mice (5/group) were harvested 2 weeks after the final immunization and re-stimulated in vitro with peptides. Shown are the percentages of IFN-γ CD8+ T cells. Data points represent individual mice. (B) Protection induced by some, but not all, Py LS DNA antigens is mediated by CD8+ T cells. Control (black bars) and experimental (gray bars) CB6F1 mice were immunized 3 times at 3 week intervals with Py DNA delivered by GG. 2 weeks after the last boost mice were challenged with 10,000 Py sporozoites intravenously and the livers were harvested 40h after the challenge. Experimental group mice were treated with anti-CD8β depleting antibody 26-28h before the challenge. Protection was defined as a significant reduction of parasite burden in the livers compared to mice immunized with EV, * P<0.05
(PDF)
CD8+ T cells were specifically depleted by intraperitoneal injection of 100μg Rat anti-mouse-CD8β antibody approximately 26-28hrs prior to challenge. (A) Representative flow plots demonstrating depletion of CD8+ T cells from PBMCs 24hrs post depletion. (B) Percent depletion of CD8+ T cells calculated compared to non-depleted control mice from the same group. Data from individual mice is shown. EV and PyCSP represent data compiled from several experiments.
(PDF)
Myc-tagged transgenic parasites were generated and designated as PyPF3D7_0730200-myc and PyPF3D7_0506200-myc. (A) Immunofluorescence of PyPF3D7_0730200-myc expressed by 24h LS Py17XNL grown in vitro. Detection of PyPF3D7_0730200-myc gene expression by Alexa-488 conjugated anti-myc antibody (green) confirms LS expression of PyPF3D7_0730200. UIS4 (red) was used as a PVM marker. (B) Immunofluorescence of PyPF3D7_0506200 expressed by 24h LS Py17XNL grown in vitro. Detection of PyPF3D7_0506200 gene expression by Alexa-594 conjugated anti-myc antibody (Red) confirms LS expression of PyPF3D7_0506200. UIS4 (green) used as a PVM marker. DAPI was used in both cases to identify nucleus. Scale bar represents 10 μm.
(PDF)
qPCR performed on RNA isolated from different parasite stages, including SS, LS at 24h, 48h, and 72h post infection, and mixed BS, to quantify the expression of each selected LS gene, normalized to expression of the parasite GAPDH gene. Graph represents box-plot of the meta-analysis of the expression rank percentile of 6 genes that protected in both parasite models (right panel) and 15 genes that failed to protect in one (n = 14) or both (n = 1) models (left panel).
(PDF)
List of primer used to verify the expression profile of selected candidate genes in SS, BS, and 24h, 48h and 72h of LS.
(PDF)
Table shows the list of primer used for generation of vaccine construct. Start codon in forward primer and stop codon in reverse primer are shown in lower case letter.
(PDF)
Table shows the list of predicted peptides from indicated antigens.
(PDF)
Table shows the primer sequence used to generate recombination construct for myc-tagging of selected Py protein. Gene ID of selected Py protein is shown in the primer name.
(PDF)
LS burden in mice vaccinated with the indicated antigen was compared to that in mice vaccinated with EV. Antigens are listed from greatest to smallest reduction in LS burden. Only antigens that significantly reduced LS burden are shown. CSP is shown in bold.
(PDF)
LS burden in mice vaccinated with a combination of CSP and the indicated antigen, was compared to that in mice vaccinated with CSP in combination with EV. Antigens are listed from greatest to smallest reduction in LS burden. Only antigens that significantly reduced LS parasite burden are shown. Doubling the CSP dose did not significantly reduce LS parasite burden beyond that achieved with CSP in combination with EV.
(PDF)
Acknowledgments
We thank Lindsay Holladay, Patricia De La Vega, Theresa Funk, Isaac Chalom, and Yogender Pal Khasa for their technical help at different steps during the antigen discovery project. We thank Dr. Dhileep Sivam for assistance in tiling microarray design. Material has been reviewed by the Walter Reed Army Institute of Research, and there is no objection to its presentation and /or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was funded in part by the PATH Malaria Vaccine Initiative, by the Intramural Research Program of NIAID, NIH and the Military Infectious Diseases Research Program of Walter Reed Army Institute of Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. (2014) Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 1005–1070. 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nussenzweig RS, Vanderberg J, Most H, Orton C (1967) Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature 216: 160–162. [DOI] [PubMed] [Google Scholar]
- 3.Clyde DF, Most H, McCarthy VC, Vanderberg JP (1973) Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci 266: 169–177. [DOI] [PubMed] [Google Scholar]
- 4.Olotu A, Fegan G, Wambua J, Nyangweso G, Awuondo KO, Leach A, et al. (2013) Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N Engl J Med 368: 1111–1120. 10.1056/NEJMoa1207564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rts SCTP (2014) Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites. PLoS Med 11: e1001685 10.1371/journal.pmed.1001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rts SCTP, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, et al. (2012) A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367: 2284–2295. 10.1056/NEJMoa1208394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casares S, Brumeanu TD, Richie TL (2010) The RTS,S malaria vaccine. Vaccine 28: 4880–4894. 10.1016/j.vaccine.2010.05.033 [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A (2010) From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin 6: 90–96. [DOI] [PubMed] [Google Scholar]
- 9.Lumsden JM, Schwenk RJ, Rein LE, Moris P, Janssens M, Ofori-Anyinam O, et al. (2011) Protective immunity induced with the RTS,S/AS vaccine is associated with IL-2 and TNF-alpha producing effector and central memory CD4 T cells. PLoS One 6: e20775 10.1371/journal.pone.0020775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foquet L, Hermsen CC, van Gemert GJ, Van Braeckel E, Weening KE, Sauerwein R, et al. (2014) Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection. J Clin Invest 124: 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. (2009) Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis 200: 337–346. 10.1086/600120 [DOI] [PubMed] [Google Scholar]
- 12.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. (2013) Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341: 1359–1365. 10.1126/science.1241800 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt NW, Butler NS, Badovinac VP, Harty JT (2010) Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog 6: e1000998 10.1371/journal.ppat.1000998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuji M, Zavala F (2003) T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol 19: 88–93. [DOI] [PubMed] [Google Scholar]
- 15.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF (1988) CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A 85: 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss WR, Jiang CG (2012) Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS One 7: e31247 10.1371/journal.pone.0031247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruner AC, Mauduit M, Tewari R, Romero JF, Depinay N, Kayibanda M, et al. (2007) Sterile protection against malaria is independent of immune responses to the circumsporozoite protein. PLoS One 2: e1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar KA, Sano G, Boscardin S, Nussenzweig RS, Nussenzweig MC, Zavala F, et al. (2006) The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 444: 937–940. [DOI] [PubMed] [Google Scholar]
- 19.Mauduit M, Tewari R, Depinay N, Kayibanda M, Lallemand E, Chavatte JM, et al. (2010) Minimal role for the circumsporozoite protein in the induction of sterile immunity by vaccination with live rodent malaria sporozoites. Infect Immun 78: 2182–2188. 10.1128/IAI.01415-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White MT, Griffin JT, Riley EM, Drakeley CJ, Moorman AM, Sumba PO, et al. (2011) Efficacy model for antibody-mediated pre-erythrocytic malaria vaccines. Proc Biol Sci 278: 1298–1305. 10.1098/rspb.2010.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, et al. (2015) Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis 211: 1076–1086. 10.1093/infdis/jiu579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogwang C, Kimani D, Edwards NJ, Roberts R, Mwacharo J, Bowyer G, et al. (2015) Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci Transl Med 7: 286re285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kester KE, Gray Heppner D Jr., Moris P, Ofori-Anyinam O, Krzych U, Tornieporth N, et al. (2014) Sequential Phase 1 and Phase 2 randomized, controlled trials of the safety, immunogenicity and efficacy of combined pre-erythrocytic vaccine antigens RTS,S and TRAP formulated with AS02 Adjuvant System in healthy, malaria naive adults. Vaccine 32: 6683–6691. 10.1016/j.vaccine.2014.06.033 [DOI] [PubMed] [Google Scholar]
- 24.Silvie O, Goetz K, Matuschewski K (2008) A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog 4: e1000086 10.1371/journal.ppat.1000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aly AS, Mikolajczak SA, Rivera HS, Camargo N, Jacobs-Lorena V, Labaied M, et al. (2008) Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol Microbiol 69: 152–163. 10.1111/j.1365-2958.2008.06271.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishino T, Boisson B, Orito Y, Lacroix C, Bischoff E, Loussert C, et al. (2009) LISP1 is important for the egress of Plasmodium berghei parasites from liver cells. Cell Microbiol 11: 1329–1339. 10.1111/j.1462-5822.2009.01333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, et al. (2008) A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A 105: 305–310. 10.1073/pnas.0710780104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guebre-Xabier M, Schwenk R, Krzych U (1999) Memory phenotype CD8(+) T cells persist in livers of mice protected against malaria by immunization with attenuated Plasmodium berghei sporozoites. Eur J Immunol 29: 3978–3986. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki LS, Gwadz RW, Godson GN (1984) Simple centrifugation method for rapid separation of sporozoites from mosquitoes. J Parasitol 70: 831–833. [PubMed] [Google Scholar]
- 30.Kaiser K, Camargo N, Kappe SH (2003) Transformation of sporozoites into early exoerythrocytic malaria parasites does not require host cells. J Exp Med 197: 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinnis P, De La Vega P, Coppi A, Krzych U, Mota MM (2013) Quantification of sporozoite invasion, migration, and development by microscopy and flow cytometry. Methods Mol Biol 923: 385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vignali M, Armour CD, Chen J, Morrison R, Castle JC, Biery MC, et al. (2011) NSR-seq transcriptional profiling enables identification of a gene signature of Plasmodium falciparum parasites infecting children. J Clin Invest 121: 1119–1129. 10.1172/JCI43457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193: 673–675. [DOI] [PubMed] [Google Scholar]
- 34.Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193. [DOI] [PubMed] [Google Scholar]
- 35.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264. [DOI] [PubMed] [Google Scholar]
- 37.Francis SE, Malkov VA, Oleinikov AV, Rossnagle E, Wendler JP, Mutabingwa TK, et al. (2007) Six genes are preferentially transcribed by the circulating and sequestered forms of Plasmodium falciparum parasites that infect pregnant women. Infect Immun 75: 4838–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386. [DOI] [PubMed] [Google Scholar]
- 39.Bergmann-Leitner ES, Leitner WW (2013) Gene gun immunization to combat malaria. Methods Mol Biol 940: 269–284. 10.1007/978-1-62703-110-3_21 [DOI] [PubMed] [Google Scholar]
- 40.Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, et al. (2009) Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol 11: 506–520. 10.1111/j.1462-5822.2008.01270.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH (2007) Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun 75: 3758–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller JL, Harupa A, Kappe SH, Mikolajczak SA (2012) Plasmodium yoelii macrophage migration inhibitory factor is necessary for efficient liver-stage development. Infect Immun 80: 1399–1407. 10.1128/IAI.05861-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estrano C, et al. (2004) A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306: 1934–1937. [DOI] [PubMed] [Google Scholar]
- 44.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF (2004) Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306: 1930–1933. [DOI] [PubMed] [Google Scholar]
- 45.Haddad D, Bilcikova E, Witney AA, Carlton JM, White CE, Blair PL, et al. (2004) Novel antigen identification method for discovery of protective malaria antigens by rapid testing of DNA vaccines encoding exons from the parasite genome. Infect Immun 72: 1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida A, Nagata T, Uchijima M, Higashi T, Koide Y (2000) Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine 18: 1725–1729. [DOI] [PubMed] [Google Scholar]
- 47.Leitner WW, Seguin MC, Ballou WR, Seitz JP, Schultz AM, Sheehy MJ, et al. (1997) Immune responses induced by intramuscular or gene gun injection of protective deoxyribonucleic acid vaccines that express the circumsporozoite protein from Plasmodium berghei malaria parasites. J Immunol 159: 6112–6119. [PubMed] [Google Scholar]
- 48.Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, Kester KE, Polhemus ME, et al. (2010) Recombinant Liver Stage Antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine 28: 5135–5144. 10.1016/j.vaccine.2009.08.046 [DOI] [PubMed] [Google Scholar]
- 49.Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, et al. (2006) A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun 74: 5933–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moorthy VS, Imoukhuede EB, Milligan P, Bojang K, Keating S, Kaye P, et al. (2004) A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med 1: e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogwang C, Afolabi M, Kimani D, Jagne YJ, Sheehy SH, Bliss CM, et al. (2013) Safety and immunogenicity of heterologous prime-boost immunisation with Plasmodium falciparum malaria candidate vaccines, ChAd63 ME-TRAP and MVA ME-TRAP, in healthy Gambian and Kenyan adults. PLoS One 8: e57726 10.1371/journal.pone.0057726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richie TL, Charoenvit Y, Wang R, Epstein JE, Hedstrom RC, Kumar S, et al. (2012) Clinical trial in healthy malaria-naive adults to evaluate the safety, tolerability, immunogenicity and efficacy of MuStDO5, a five-gene, sporozoite/hepatic stage Plasmodium falciparum DNA vaccine combined with escalating dose human GM-CSF DNA. Hum Vaccin Immunother 8: 1564–1584. 10.4161/hv.22129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fidock DA, Gras-Masse H, Lepers JP, Brahimi K, Benmohamed L, Mellouk S, et al. (1994) Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol 153: 190–204. [PubMed] [Google Scholar]
- 54.Sedegah M, Hedstrom R, Hobart P, Hoffman SL (1994) Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci U S A 91: 9866–9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Limbach K, Aguiar J, Gowda K, Patterson N, Abot E, Sedegah M, et al. (2011) Identification of two new protective pre-erythrocytic malaria vaccine antigen candidates. Malar J 10: 65 10.1186/1475-2875-10-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aly AS, Lindner SE, MacKellar DC, Peng X, Kappe SH (2011) SAP1 is a critical post-transcriptional regulator of infectivity in malaria parasite sporozoite stages. Mol Microbiol 79: 929–939. 10.1111/j.1365-2958.2010.07497.x [DOI] [PubMed] [Google Scholar]
- 57.Raj DK, Nixon CP, Nixon CE, Dvorin JD, DiPetrillo CG, Pond-Tor S, et al. (2014) Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science 344: 871–877. 10.1126/science.1254417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berenzon D, Schwenk RJ, Letellier L, Guebre-Xabier M, Williams J, Krzych U (2003) Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J Immunol 171: 2024–2034. [DOI] [PubMed] [Google Scholar]
- 59.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V (1987) Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330: 664–666. [DOI] [PubMed] [Google Scholar]
- 60.Malik A, Egan JE, Houghten RA, Sadoff JC, Hoffman SL (1991) Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci U S A 88: 3300–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Meta-analyses of 5 independent immunization experiments and resulting LS parasite burden reduction in Py model with IM immunization. (B) Meta-analyses of 7 independent immunization experiments and resulting LS parasite burden reduction in Py model induced by EP immunizations. (C) Meta-analyses of 4 independent immunization experiments and resulting LS parasite burden reduction in Pb model induced by EP immunizations. Each square or triangle represents one BALB/c or B6 mouse, respectively. Green color indicates significant difference as compared to EV immunized groups tested in the same immunization studies (p<0.05). Red color indicates p>0.05 and therefore no significant difference in LS burden as compared to EV immunized mice. CSP (purple) was used as positive control. EV (black) was used as negative control.
(PDF)
(A) Three of four novel antigens delivered as DNA vaccines induced IFN-γ responses recalled in CD8+ T cells by peptides predicted to be CD8+ T cell epitopes for either H-2b or H-2d mouse haplotypes. Spleens from immunized CB6F1 mice (5/group) were harvested 2 weeks after the final immunization and re-stimulated in vitro with peptides. Shown are the percentages of IFN-γ CD8+ T cells. Data points represent individual mice. (B) Protection induced by some, but not all, Py LS DNA antigens is mediated by CD8+ T cells. Control (black bars) and experimental (gray bars) CB6F1 mice were immunized 3 times at 3 week intervals with Py DNA delivered by GG. 2 weeks after the last boost mice were challenged with 10,000 Py sporozoites intravenously and the livers were harvested 40h after the challenge. Experimental group mice were treated with anti-CD8β depleting antibody 26-28h before the challenge. Protection was defined as a significant reduction of parasite burden in the livers compared to mice immunized with EV, * P<0.05
(PDF)
CD8+ T cells were specifically depleted by intraperitoneal injection of 100μg Rat anti-mouse-CD8β antibody approximately 26-28hrs prior to challenge. (A) Representative flow plots demonstrating depletion of CD8+ T cells from PBMCs 24hrs post depletion. (B) Percent depletion of CD8+ T cells calculated compared to non-depleted control mice from the same group. Data from individual mice is shown. EV and PyCSP represent data compiled from several experiments.
(PDF)
Myc-tagged transgenic parasites were generated and designated as PyPF3D7_0730200-myc and PyPF3D7_0506200-myc. (A) Immunofluorescence of PyPF3D7_0730200-myc expressed by 24h LS Py17XNL grown in vitro. Detection of PyPF3D7_0730200-myc gene expression by Alexa-488 conjugated anti-myc antibody (green) confirms LS expression of PyPF3D7_0730200. UIS4 (red) was used as a PVM marker. (B) Immunofluorescence of PyPF3D7_0506200 expressed by 24h LS Py17XNL grown in vitro. Detection of PyPF3D7_0506200 gene expression by Alexa-594 conjugated anti-myc antibody (Red) confirms LS expression of PyPF3D7_0506200. UIS4 (green) used as a PVM marker. DAPI was used in both cases to identify nucleus. Scale bar represents 10 μm.
(PDF)
qPCR performed on RNA isolated from different parasite stages, including SS, LS at 24h, 48h, and 72h post infection, and mixed BS, to quantify the expression of each selected LS gene, normalized to expression of the parasite GAPDH gene. Graph represents box-plot of the meta-analysis of the expression rank percentile of 6 genes that protected in both parasite models (right panel) and 15 genes that failed to protect in one (n = 14) or both (n = 1) models (left panel).
(PDF)
List of primer used to verify the expression profile of selected candidate genes in SS, BS, and 24h, 48h and 72h of LS.
(PDF)
Table shows the list of primer used for generation of vaccine construct. Start codon in forward primer and stop codon in reverse primer are shown in lower case letter.
(PDF)
Table shows the list of predicted peptides from indicated antigens.
(PDF)
Table shows the primer sequence used to generate recombination construct for myc-tagging of selected Py protein. Gene ID of selected Py protein is shown in the primer name.
(PDF)
LS burden in mice vaccinated with the indicated antigen was compared to that in mice vaccinated with EV. Antigens are listed from greatest to smallest reduction in LS burden. Only antigens that significantly reduced LS burden are shown. CSP is shown in bold.
(PDF)
LS burden in mice vaccinated with a combination of CSP and the indicated antigen, was compared to that in mice vaccinated with CSP in combination with EV. Antigens are listed from greatest to smallest reduction in LS burden. Only antigens that significantly reduced LS parasite burden are shown. Doubling the CSP dose did not significantly reduce LS parasite burden beyond that achieved with CSP in combination with EV.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.