Figure 1.
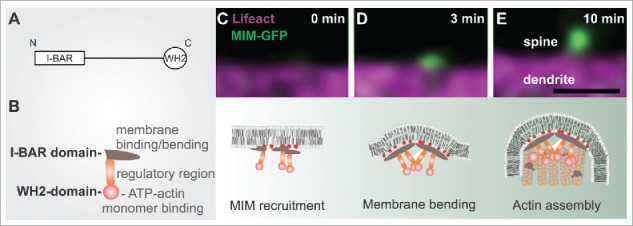
The role of MIM during dendritic filopodia initiation. (A) MIM domain structure. (B) A schematic representation of an MIM monomer, which dimerizes via its I-BAR domain. The central region contains polyproline stretches and sites that are modified post-translationally. The C-terminus contains an ATP-actin binding WH2 domain. (C-E) The upper panel displays time frames of initiating dendritic filopodia and below is a schematic summary of each stage according to our findings.3 (C) phosphoinositide-dependent recruitment of MIM results in its site-specific oligomerization and (D) outward membrane bending. (E) The subsequent elongation of the protrusion requires Arp2/3-mediated actin assembly. Scale bar 0.5 μm.
