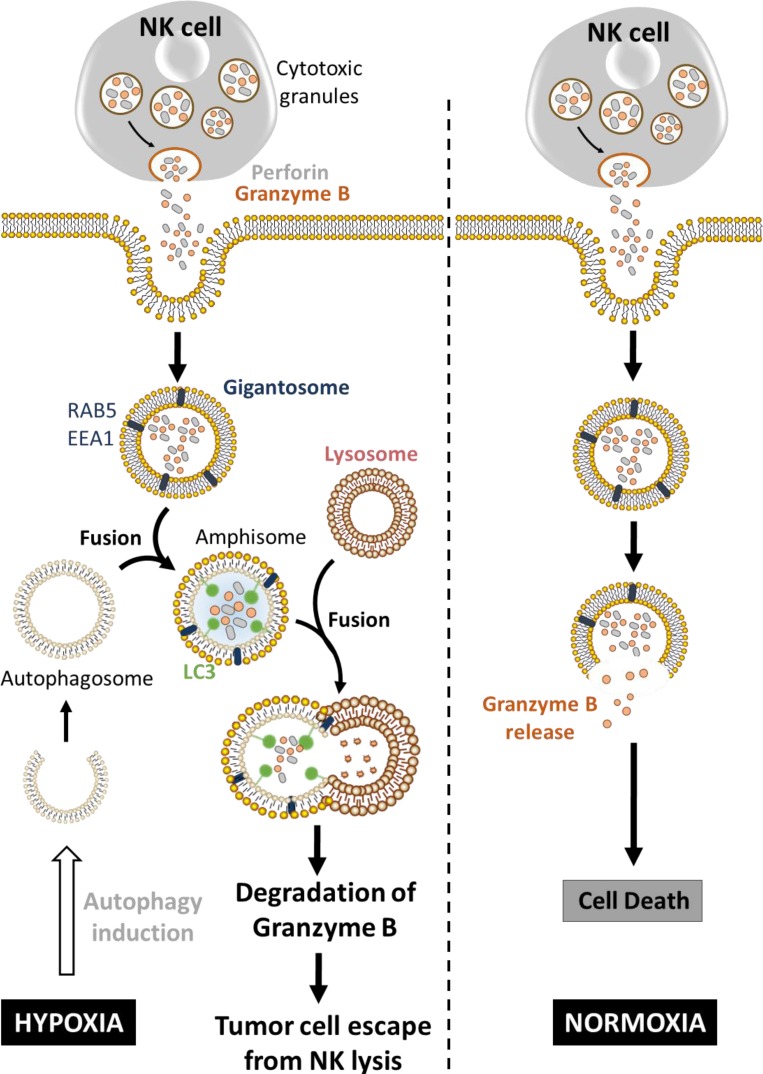
Figure 3. Hypoxia-induced autophagy degrades NK-derived granzyme B and impairs NK-mediated killing.
Following recognition by NK cells, the cytolytic granules containing perforin and granzyme B enter the target cells through endocytosis and traffic to enlarged endosomes called “gigantosomes” characterized by the expression of endosome markers (RAB5 or EEA1). In normoxic cells, perforin forms pores in the “gigantosome” membrane, allowing granzyme B release and the initiation of cell death. In hypoxic cells, excessive autophagy leads to the fusion of “gigantosomes” with autophagosomes and the subsequent formation of amphisomes containing granzyme B and perforin. The fusion of amphisome with lysosome triggers the selective degradation of granzyme B, making hypoxic tumor cell less sensitive to NK-mediated killing.