Figure 1. DNA origin activation checkpoint.
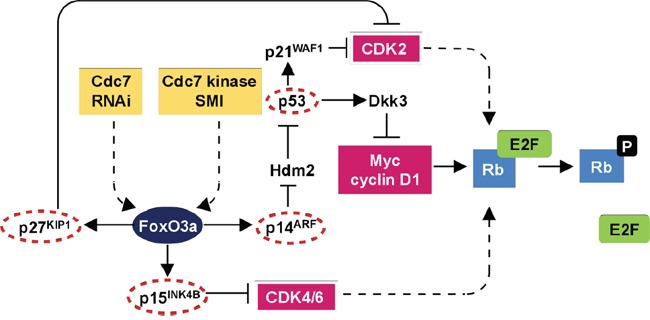
The checkpoint response is dependent on three effector axes coordinated through the transcription factor FoxO3a. In arrested cells, FoxO3a activates the ARF-|Hdm2-|p53→p21 pathway and mediates p15(INK4B) upregulation. p53 in turn activates expression of the Wnt/β-catenin signalling antagonist Dkk3, leading to Myc and cyclin D1 downregulation. The resulting loss of CDK activity inactivates the Rb-E2F pathway resulting in G1 arrest. The lack of redundancy between the checkpoint axes and reliance on several tumour suppressor proteins commonly inactivated in human tumours provides a mechanistic basis for the cancer-cell-specific killing observed following targeting of Cdc7. Notably multiple core components of this checkpoint pathway are mutated in pancreatic cancer (circled in red) thus sensitizing this tumour type to Cdc7 directed agents (adapted from Tudzarova et al, EMBO J. 2010 Oct 6;29(19):3381-94, Figure 6).
