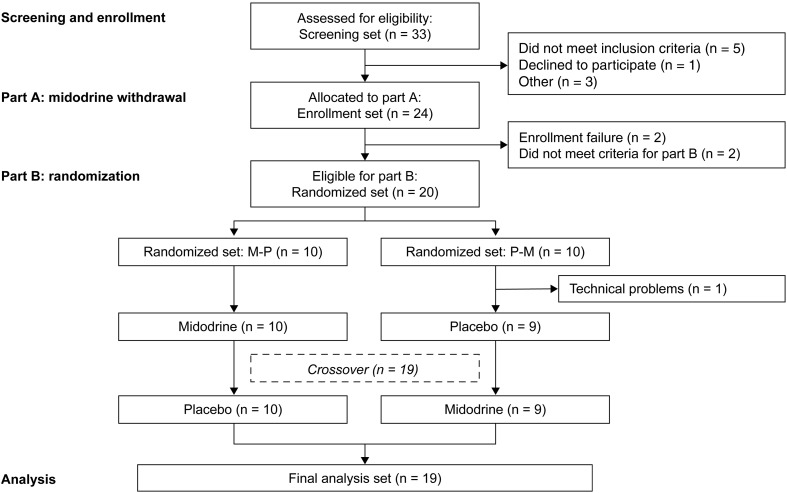
Fig. 2.
Participant flow. Thirty-three patients were screened for inclusion in the study and participated in the assessments on day −1 and day 1; of these, nine did not meet the inclusion criteria. The enrollment set comprised 24 participants. Four patients were withdrawn from the study during Part A. The randomized set comprised the 20 participants who received at least one dose of midodrine in Part B. One participant did not complete the study because of technical problems with the tilt-table on day 2 and was excluded from the final analysis set