Abstract
Over the last few years, vitamin D deficiency has emerged as a risk factor for many diseases. Public awareness of the importance of the ‘sunshine vitamin’ is increasing, however deficiency remains an ongoing problem. Is an awareness of the importance of vitamin D enough to promote healthy people to take supplements or is a different approach required? In this article the importance of vitamin D is discussed and data showing that knowledge of this is not sufficient to encourage people to take supplements are presented.
Key Words: asthma, cardiovascular disease, dietary supplementation, multiple sclerosis, tuberculosis, vitamin D
Vitamin D deficiency has emerged as a risk for many diseases–multiple sclerosis,1 asthma, rheumatoid arthritis, cardiovascular disease and tuberculosis (TB), to name but a few. While vitamin D has long been known to have effects on skeletal and renal tissues, the cognate receptor for vitamin D, the vitamin D receptor, is present in a wide variety of tissues and cells, including circulating T- and B-lymphocytes and antigen-presenting cells, such as macrophages and dendritic cells. Immune cells are able to transform 25-hydroxyvitamin D into the active metabolite, 1,25-dihydroxyhydroxyvitamin D, which in turn exerts auto- and paracrine effects on the same cells and their neighbours.2
As 1,25-hydroxyvitamin D has a half-life of hours and is regulated by parathyroid hormone (PTH) and calcium/phosphate levels, serum 25-hydroxyvitamin D levels are widely accepted as being representative of overall vitamin D stores and availability. Serum levels above 75 nmol/l are considered ‘sufficient’.3 At levels below 75 nmol/l, calcium absorption by the gut is suboptimal and PTH secretion is stimulated.1 However, in the wider range of diseases for which low serum vitamin D is a significant risk factor, little work has been done to determine the optimal vitamin D levels.
The controversies surrounding vitamin D supplementation in clinical practice were recently highlighted in the New England Journal of Medicine.3 Optimal levels of vitamin D to maintain skeletal health may well be different from those indicated for other diseases.3 Studies examining the effect of vitamin D on osteoporosis have shown inconsistent results, with the overall picture being of slight benefit with little risk.4 Trials of vitamin D supplementation as an adjunct to antituberculous therapy in those with active TB have not shown a benefit in terms of either mortality or clinical outcome.5 However, given the lack of knowledge regarding optimal dosage, this outcome is far from conclusive. No randomised controlled trials of vitamin D supplementation have been carried out in any of the other non-skeletal diseases associated with deficiency, although momentum is gathering.
The epidemic of vitamin D deficiency in both the UK6 and other countries of similar latitude,7 is well described through large, population-based epidemiological studies. More recently, this has reached a wider audience, with interest in vitamin D deficiency increasing in the popular press. With interest in the ‘sunshine vitamin’ increasing, are those of us with a vested interest in vitamin D levels heeding our own advice?
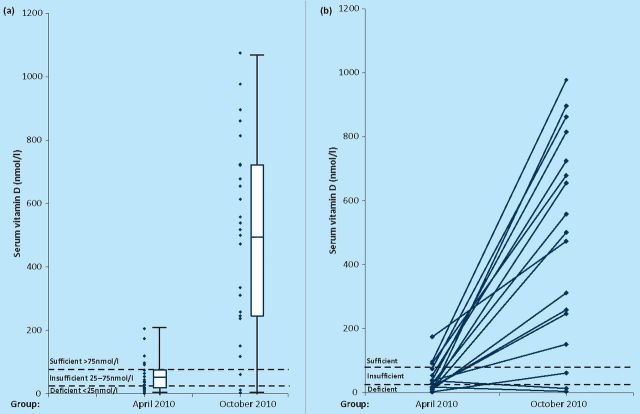
The authors work in a research group where one focus of work examines the potential link between vitamin D deficiency and multiple sclerosis. Among the group the awareness of the link between low vitamin D and disease is very high; indeed the research blog from the group advocates supplementation of up to 5,000 IU per day (http://multiple-sclerosis-research.blogspot.com/2010/02/what-dose-of-vitamin-d.html). One would suppose, therefore, that members of the group would take steps to ensure that sun exposure and vitamin D intake levels were sufficient, especially over the winter months. In order to test this hypothesis, members of the research group were studied at the end of winter (April 2010, n=23) and the end of summer (October 2010, n=27). Serum 25-hydroxyvitamin D levels were then determined using a commercially available ELISA (Immundiagnostik, Germany; ref K2106). As can be seen in Fig 1, the difference between end of winter and end of summer serum vitamin D levels is easily apparent (p=1.35×10∘−8, t-test on log values). At the end of winter, 77.8% of subjects did not have sufficient vitamin D levels, whereas this proportion fell to 12.0% at the end of summer. This differs from a UK population-based study,6 where 87.1% and 60.9% were not sufficient at the end of winter and summer respectively.
Fig 1.
(a) Combined scatter and box-and-whisker plot showing serum 25-hydroxyvitamin D levels (nmol/l) at the end of winter (April 2010) and end of summer (October 2010). The box indicates the mean +/− 1 standard deviation, and the whiskers the range. The dashed lines indicate the accepted levels for deficiency, insufficiency and sufficiency (<25 nmol/l, 25–75 nmol/l and >75 nmol/l respectively), p=1.35×10∘−8 for a difference between the two groups. (b) Graph showing the change in serum 25-hydroxyvitamin D levels (nmol/l) between the end of winter (April 2010) and end of summer (October 2010) between paired samples (ie the same subjects, rather than matched pairs, therefore not all subjects shown).
It would appear, therefore, that a relatively detailed knowledge of the importance of adequate serum vitamin D levels is insufficient to motivate those ‘in the know’ to take sufficient vitamin D supplements over the winter months. The proportion of subjects taking any vitamin D supplementation actually fell over the summer months, from 39% supplementing at the end of winter to 22% at the end of summer (difference not statistically significant). However, those taking supplements were more likely to do so regularly at the end of summer, probably due to informal discussions about the preliminary results of this study (7.41% regularly supplementing at the end of winter ν 34.78% at the end of summer, p = 0.02). In those taking supplements there was a wide variation in the dose taken, from 400 IU to 5,000 IU/day.
These observations are timely, particularly in view of the current debate on what is the most suitable recommended daily allowance for vitamin D supplementation. The Institute of Medicine has recently published guidelines on recommended vitamin D intake, which recommend a daily intake of around 600 IU, with a safe maximum of 4,000 IU.8 The use of vitamin D supplements has in the past been clouded by concerns regarding safety.9 Clearly the current recommended dietary allowance for vitamin D supplementation is inadequate and this important issue needs to be addressed.
What hope is there for patients and the general population? The guidelines surrounding vitamin D supplementation are constantly changing, have a wide variety of recommended doses, and appear to have little in the way of rigorous scientific background. When attempting to supplement the diet of healthy people, compliance issues are a major barrier. One possible strategy to avoid these could be the fortification of foodstuffs, although this clearly has major ethical and practical implications.
At the moment, therefore, an attempt to improve education about the importance of vitamin D, together with further research into its role in health and disease is a necessity. Awareness surrounding vitamin D is already on the increase, and it is hoped that this will be maintained, and translated into rigorous public health strategies.
Ethical approval
This study received ethical approval from the Oxfordshire Research Ethics committee C, ref 05/Q1601/76.
References
- 1.Pierrot-Deseilligny C, Souberbielle JC. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain 2010; 133: 1869–88 10.1093/brain/awq147 [DOI] [PubMed] [Google Scholar]
- 2.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010; 10: 482–96 10.1016/j.coph.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 3.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med 2011; 364: 248–54 [DOI] [PubMed] [Google Scholar]
- 4.Avenell A, Gillespie WJ, Gillespie LD, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev 2009CD000227. [DOI] [PubMed] [Google Scholar]
- 5.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2009; 179: 843–50 10.1164/rccm.200804-567OC [DOI] [PubMed] [Google Scholar]
- 6.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 2007; 85: 860–8 [DOI] [PubMed] [Google Scholar]
- 7.Langlois K, Greene-Finestone L, Little J, Hidiroglou N, Whiting S. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep 2010; 21: 47–55 [PubMed] [Google Scholar]
- 8.Institute of Medicine. Dietary reference intakes for calcium and vitamin D 2010Washington DC: National Academy Press [Google Scholar]
- 9.Cranney A, Horsley T, O'Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess 20071–235 [PMC free article] [PubMed] [Google Scholar]