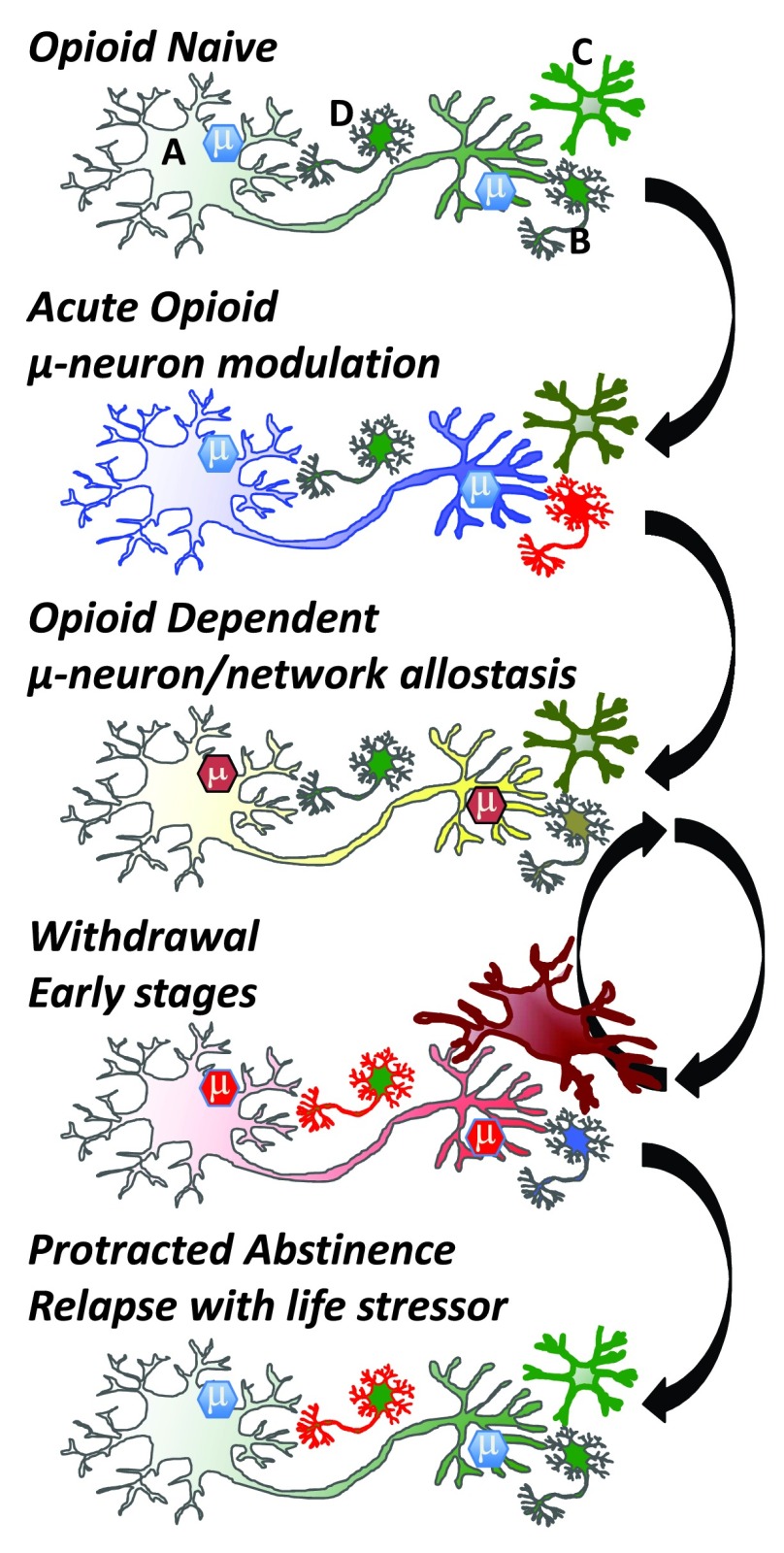
Figure 3. Cellular adaptations to opioids.
In the ‘Opioid Naïve’ state, mu opioid receptor expressing cells (e.g., GABAergic neuron; top panel, neuron A) modulate reward circuitry and many other neurons (top panel, neuron B) in the brain and periphery. Brain microglia (top panel, cell C) are normally in a quiescent surveillance state. Acute opioid administration activates mu opioid receptors, which couple to inhibitory G proteins and generate an active mu-signalosome (μ) that inhibits cell activity and neurotransmitter release. In the brain, mu opioid receptors are often on GABAergic inhibitory neurons and thus can activate adjacent neurons (B) via disinhibition. Microglia may also be activated directly by opioid drugs, although this is debated. In the ‘Opioid Dependent’ state, opioid receptor expressing neurons adapt to the continued presence of drug. Modifications occur in the µ-signalosome, neuronal proteome, and transcriptome, alongside modifications to neuronal morphology (e.g., spine density and dendritic arborization). Maintained activation of mu-opioid receptors begins a process of cellular, network, and system adaptations. On drug cessation withdrawal is triggered, and the adaptive (allostatic) changes that occur in the drug-dependent state rebound in a temporally (hours to years) orchestrated resetting of neurons and networks. Withdrawal symptoms are opposing the acute actions of opioids whereby neurons inhibited by opioid activation become excited during withdrawal. Other cells are engaged during withdrawal, including microglia (Cell C) and neurons within the anxiogenic learning circuitry (Cell D). Eventually, weeks to months after drug cessation, many networks re-establish a close-to-normal state, but neurons encoding memory circuits of withdrawal (cell D) and associated memories that drugs relieve aversive states can be triggered by stressful or aversive life events following ‘Protracted Abstinence’. Green: resting state, blue: inhibited state, yellow: near-normal dependent state, red: activated state. Arrows denote transition between states.