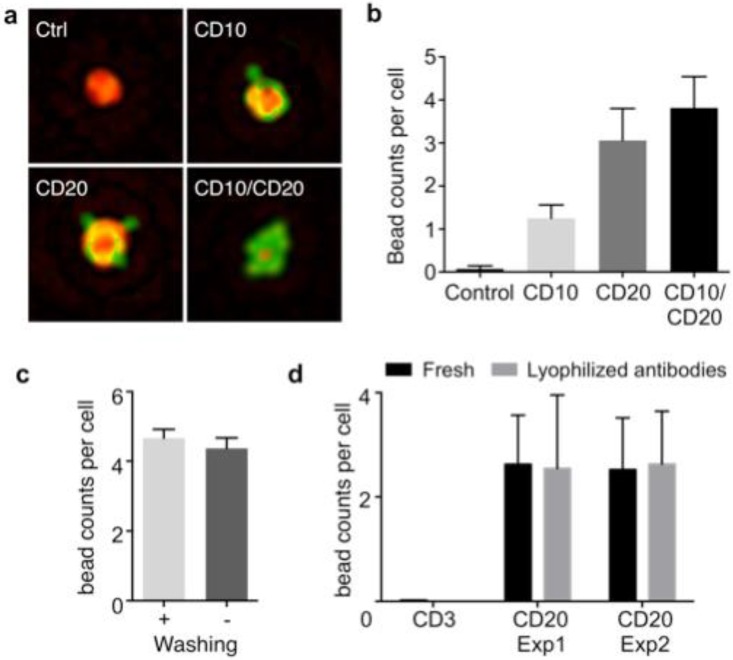
Figure 2.
Optimization of HALT platform for high-throughput analyses. (a) Composite images of cells immunolabeled with microbeads (4.5 μm) and imaged using HALT. The amplitude (green) and the phase (red) were pseudocolored for clarification. (b) Using an antibody cocktail improves HALT sensitivity. The average number of beads per cell is higher in the presence of cocktail of CD10 and CD20 antibodies as compared to CD10 or CD20 used individually in DB cells. (c) The robust specificity of the system can help in decreasing processing times by omitting the washing steps. No decrease in the signal was observed for CD20 antibody without washing the Daudi cells' samples. (d) In order to increase the portability and shelf-life of our system we tested the system with lyophilized antibodies. Lyophilized antibodies (CD3 and CD20) were tested and compared with parent solution antibodies after 2 weeks of storage at 4℃ by HALT platform and exhibited similar activity as the original antibodies (Exp1: experiment 1, Exp:2 experiment 2 show data from antibodies lyophilized at two different occasions).