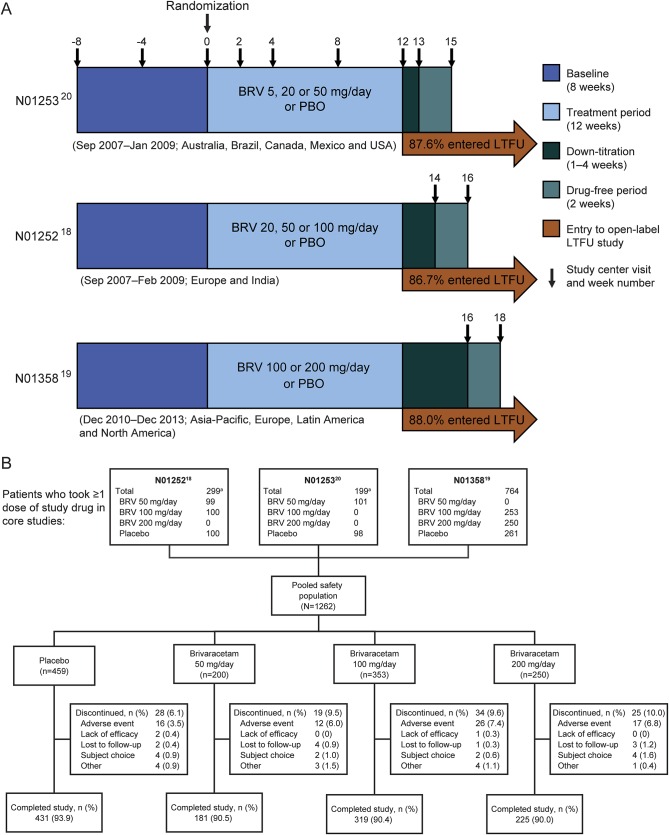
Figure 1. Study designs and patient disposition.
(A) Designs of phase III fixed-dose studies N01253,20 N01252,18 and N01358.19 Concomitant levetiracetam was limited to ≤20% of patients in studies N01252 and N01253 and was not permitted in study N01358. (B) Patient disposition (safety population). aPatients taking BRV 5 or 20 mg/d were excluded from the pooled analysis. BRV = brivaracetam; LTFU = long-term follow-up; PBO = placebo.