Fig. 4. Tyrosine kinase activation of nucleotide exchange modulates KRASG12C inhibition in cancer cells.
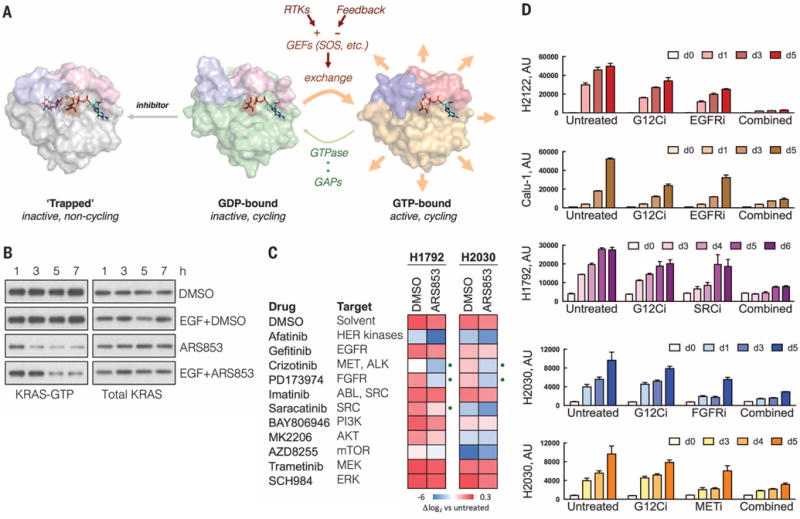
(A) A schematic of the trapping mechanism by which ARS853 targets KRASG12C-dependent signaling and the role of RTK- or feedback-regulated nucleotide exchange in this process. Structures were adapted from Protein Data Bank accessions 4luc (inhibitor-bound KRASG12C-GDP), 4ldj (KRASG12C-GDP), and 4l9w (HRASG12C-GTP). (B) H358 cells were serum starved overnight followed by treatment with ARS853 (10 μM), with or without EGF (100 ng/ml) for the indicated times, to determine the effect on KRAS-GTP. A representative of two independent experiments is shown. (C) The indicated cell lines, selected because of their relative insensitivity to KRASG12C inhibition, were treated with inhibitors of RTK signaling, alone or in combination with ARS853 for 5 days, in order to determine the most effective combinations. The effect of treatment is shown relative to the proliferation of untreated cells. The dots indicate combination treatments resulting in significantly more pronounced inhibition, compared to either drug alone (n = 3 replicates, mean). DMSO, dimethyl sulfoxide. (D) The effect of inhibitors targeting tyrosine kinases on the antiproliferative effect of KRASG12C inhibition in KRASG12C mutant cell lines (n = 3 replicates, mean and SEM). The concentrations of the inhibitors used in (C) and (D) are noted in Materials and Methods.
