Figure 12.
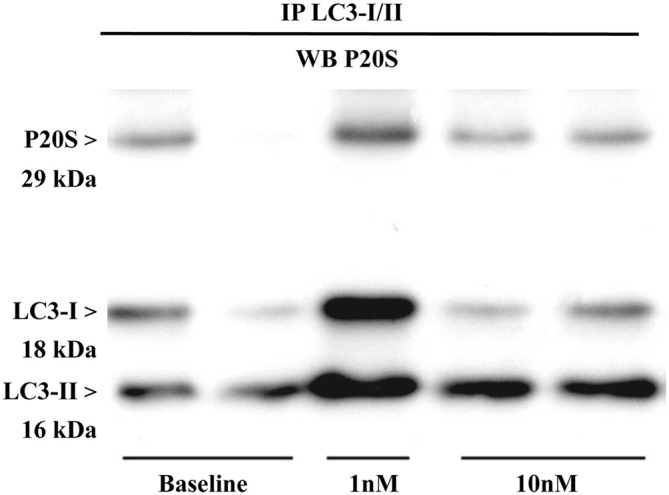
Immunoprecipitation with anti-LC3 antibody and immunoblotting with anti-P20S. P20S is clearly detected by western blotting carried out from LC3-immunoprecipitates from two baseline conditions and following two doses of rapamycin (one for 1 nM and two for 10 nM). P20S is present in baseline conditions and following rapamycin. This strongly suggests the occurrence of a molecular binding between LC3 and P20S. The amount of P20S may either increase or decrease depending on how much it is dragged down by LC3, which in turn depends on direct or indirect binding between P20S and LC3 itself which occurs in each experimental conditions. In fact, the intensity of the P20S band does not correspond to P20S particles but it rather represent P20S particles bound to LC3. This phenomenon suggests that the amount of binding between LC3 and P20S decreases when compared with the increase in LC3 and P20S particles per se. This is likely to depend on the fact that despite increasing P20S and LC3, mTOR inhibition also promotes the enzymatic conversion of those structures bridging LC3 with P20S as reported in the “Discussion” Section. The representative blotting shows a rapamycin-dependent increase in the ratio between LC3-II and LC3-I which is consistent with rapamycin-induced ATG activation as shown all over the article.
