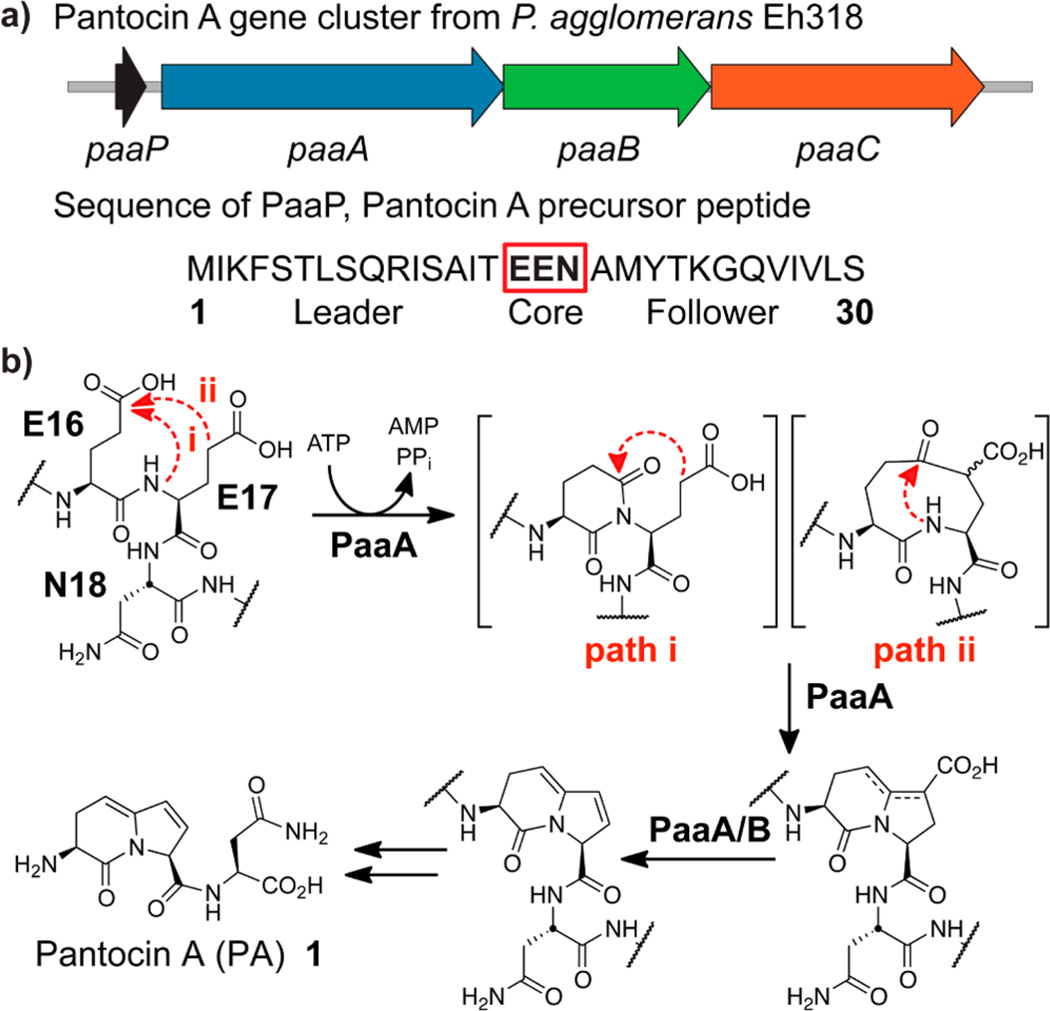
Figure 1.
(a) Pantocin (PA) biosynthetic cluster from P. agglomerans and (b) proposed biosynthesis of Pantocin A. Biosynthesis requires condensation onto the peptide backbone and Claisen-like condensation between the two glutamic acids. Logically, either condensation (path i or ii) could occur first and either glutamate residue could provide the putative enolate nucleophile. A subsequent oxidative decarboxylation will yield the same bicyclic core. A detailed mechanistic proposal is given in Scheme S1 in the Supporting Information.