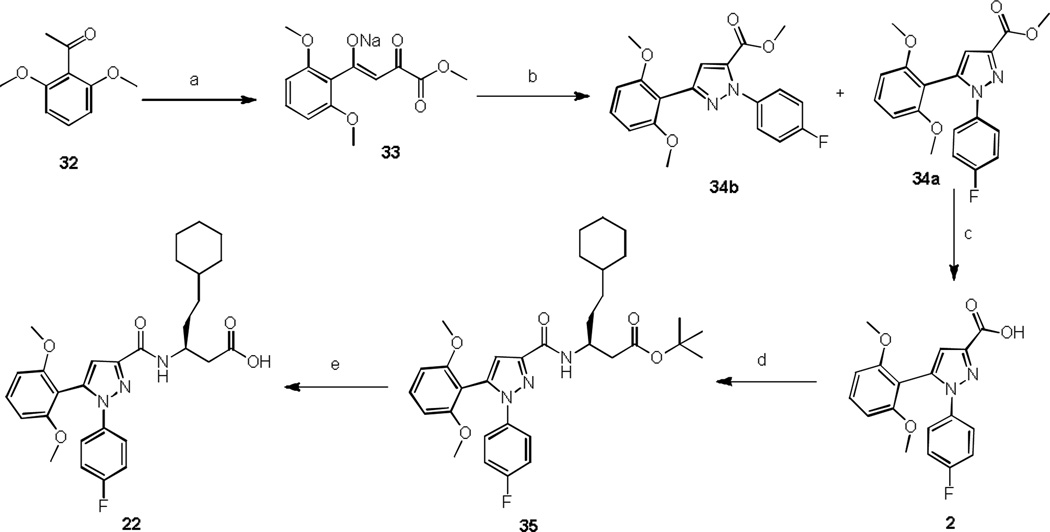
Scheme 1.
Synthesis of compound 22
Reagents and conditions: a) Diethyl oxalate, MeONa, MeOH, reflux, 6 h, 90%; b) 4-Fluorophenyl hydra-zine hydrochloride, glacial acetic acid, conc. HCl, reflux, 3.5 h, 61%; c) LiOH, MeOH/THF/H2O, rt, 18 h, 87%; d) Tert-butyl (S)-3-amino-5-cyclohexylpentanoate, HBTU, Et3N, CH3CN, rt, 3 h, 79%; e) TFA, DCM, rt, 4 h, 80%