Abstract
Objective
Faster time from onset to recanalization (OTR) in acute ischemic stroke using endovascular therapy (ET) has been associated with better outcome. However, previous studies were based on less-effective first-generation devices, and analyzed only dichotomized disability outcomes, which may underestimate the full effect of treatment.
Methods
In the combined databases of the SWIFT and STAR trials, we identified patients treated with the Solitaire stent retriever with achievement of substantial reperfusion (Thrombolysis in Cerebral Infarction [TICI] 2b–3). Ordinal numbers needed to treat values were derived by populating joint outcome tables.
Results
Among 202 patients treated with ET with TICI 2b to 3 reperfusion, mean age was 68 (±13), 62% were female, and median National Institutes of Health Stroke Scale (NIHSS) score was 17 (interquartile range [IQR]: 14– 20). Day 90 modified Rankin Scale (mRS) outcomes for OTR time intervals ranging from 180 to 480 minutes showed substantial time-related reductions in disability across the entire outcome range. Shorter OTR was associated with improved mean 90-day mRS (1.4 vs. 2.4 vs. 3.3, for OTR groups of 124–240 vs. 241–360 vs. 361–660 minutes; p < 0.001). The number of patients identified as benefitting from therapy with shorter OTR were 3-fold (range, 1.5–4.7) higher on ordinal, compared with dichotomized analysis. For every 15-minute acceleration of OTR, 34 per 1,000 treated patients had improved disability outcome.
Interpretation
Analysis of disability over the entire outcome range demonstrates a marked effect of shorter time to reperfusion upon improved clinical outcome, substantially higher than binary metrics. For every 5-minute delay in endovascular reperfusion, 1 of 100 patients has a worse disability outcome.
Endovascular therapy (ET) has emerged as the first major advance in acute ischemic stroke (AIS) care in nearly two decades. A series of recent studies is establishing a clear improvement in ultimate clinical outcome for patients treated with this technique against best medical care alone.1–4 However, time from onset to reperfusion (OTR) is a key modifier of treatment benefit.
The observation that shorter time to reperfusion is associated with better outcome was first established for treatment with intravenous fibrinolysis.5–7 Several recent studies have identified OTR as an important determinant as well of binary functional outcomes after endovascular intervention.8–10 For example, one study noted a nearly 11% decrease in likelihood of achieving a nondisabled outcome (defined as 90-day modified Rankin scale [mRS] of 0–2 vs. 3–6) with a 30-minute delay.8 However, by focusing only on dichotomized functional endpoints, these previous analyses are likely to have substantially underestimated the influence of OTR on improving clinical outcome. Reperfusion is likely to improve outcomes across the entire range of disability, and focusing on health-state transitions at only a single point in this range provides a highly incomplete index of therapy effects.11–13 In this study, we seek to determine the full impact of OTR on clinical outcomes, by examining its influence over the full 7-value modified Rankin disability scale.
In addition, previous analyses focused on patients treated with previous generation and less-effective ET approaches, such as intra-arterial fibrinolysis and coil retrievers. These techniques are representative of the initial round of ET trials that failed to demonstrate benefit over medical therapy, and, as such, their data cannot be directly applied to current techniques that are stent-retriever based. Thus, in order to obtain results most compatible with modern trials and current clinical practice, we performed this analysis in a combined cohort of two large stent-retriever based studies, the SWIFT and STAR trials.14,15
Subjects and Methods
Study Design and Participants
SWIFT was a multicenter, randomized, prospective, parallel-group trial with blinded primary endpoint ascertainment. Details of the study design are available elsewhere.14 The STAR trial was an international, prospective, multicenter, single-arm study.15 Briefly, for both studies, patients were eligible if they had acute ischemic stroke with moderate-to-severe neurological deficits, harbored angiographically confirmed occlusions of proximal cerebral arteries, and were treatable by thrombectomy within 8 hours of stroke symptom onset. Key inclusion criteria included age (22–85 years in SWIFT, 18–85 in STAR), National Institutes of Health Stroke Scale (NIHSS) score 8–30, and ineligibility for, or failure to respond to, intravenous recombinant tissue-type plasminogen activator (rt-PA) with documented occlusion of an anterior intracranial artery. Key exclusion criteria included uncontrolled hypertension, serious sensitivity to radiographical contrast agents, and computed tomography (CT) or magnetic resonance imaging evidence of intracranial hemorrhage or major ischemic infarction (acute ischemic change in more than one third of the middle cerebral artery territory or more than 100mL of tissue in other territories). The studies were approved by the appropriate national regulatory bodies and by the ethics committee at each center. All patients or their legally authorized representatives provided signed informed consent.
Procedures
In the SWIFT trial, once enrolled, patients were treated with Solitaire stent-retriever device (roll-in phase) or randomized to treatment with the Solitaire stent-retriever device or the Merci device (randomized phase). All patients in the STAR study were treated with the Solitaire device. In this analysis, we included only patients treated with the Solitaire device for anterior circulation occlusions (internal carotid artery [ICA] or middle cerebral artery), who achieved substantial reperfusion, defined as Thrombolysis in Cerebral Infarction (TICI) scale scores of 2b or 3, as determined by a blinded core laboratory assessment.16 Time to reperfusion was defined as the time from when the patient was last known to be well until the visualization of successful reperfusion as defined above in all treatable vessels. Global disability at 3 months was assessed with the 7-level modified Rankin Scale (mRS) in both studies.
Statistical Analysis
Key statistical analyses, including the primary endpoint analysis, were validated by an independent external statistician (J. Schafer, MS, NAMSA, Minneapolis, MN). Analyses of continuous variables were calculated by t test (when mean is reported) or Wilcoxon test (when median is reported). Analyses of discrete variables were conducted using Fisher’s exact test. Onset to reperfusion was analyzed both as a continuous variable and as a trichotomized one with groupings of 120 to 240, 241 to 360, and 361 to 660 minutes. Multivariate analysis of factors influencing OTR was performed using linear regression. Adjusted ordinal logistic regression was used to model the effect of OTR on 90-day outcomes across the entire distribution of mRS. This analysis was adjusted for the following variables: age, sex, baseline NIHSS value, age × NIHSS interaction term, history of atrial fibrillation, history of hypertension, history of coronary artery disease or myocardial infarction, history of diabetes, history of hyperlipidemia, history of peripheral vascular disease, smoking, previous stroke or transient ischemic attack (TIA), prestroke Rankin Scale, target occlusion location, baseline serum glucose, and ASPECTS.17 For dichotomous outcomes, number needed to treat to benefit (NNT) values were derived by dividing 100 by the absolute risk reduction. For ordinal outcomes, the NNT values were derived using algorithmic population of the joint outcome table.18 In sensitivity analysis, these values were derived using two additional joint outcome table population methods: the permutation test and a bootstrapped automated random sampling method.18,19 Benefit per thousand (BPT) values were obtained by multiplying the inverse of NNT by 1,000.
Results
Among the 292 patients allocated to Solitaire treatment in the SWIFT and STAR studies, 202 (69%) achieved successful reperfusion for anterior circulation occlusions and met the inclusion criteria for this analysis. Substantial reperfusion in the anterior circulation was achieved in 84.2% (160 of 190) patients in the STAR study and 81% (42 of 52) in the SWIFT study by core lab assessments. Among these 202 patients, mean age was 68 (±13), 62% were female, median NIHSS was 17 (interquartile range [IQR], 14–20), and premorbid mRS was 0 in 71% and 1 in 16%.
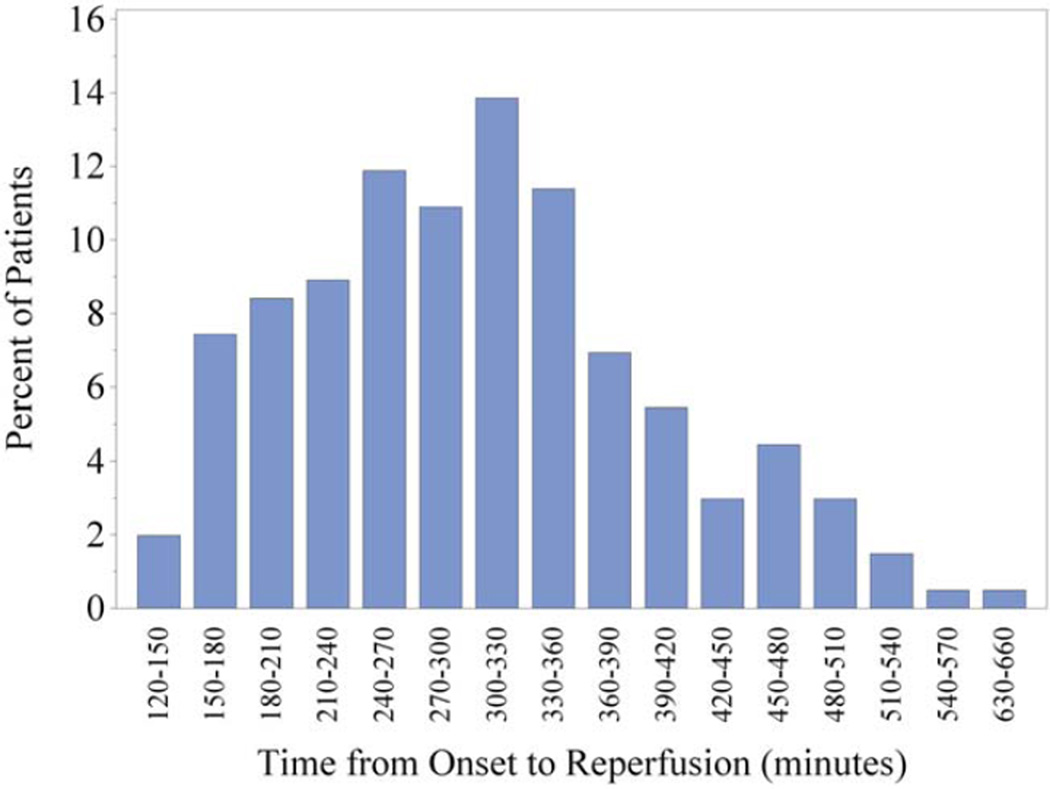
As shown in Table 1, baseline clinical and demographic characteristics were generally similar for patients with onset-to-reperfusion times (OTR) in the 2- to 4-, 4 to 6-, and 6- to 11-hour time windows. Notable differences include a trend toward slightly increased stroke severity in the patients in the longest time window (NIHSS 17), compared to the earlier ones (NIHSS 15). There was an increased frequency of diabetes in the patients in the longest time window as well as a trend toward higher baseline serum glucose levels on arrival in the patients in the later time windows. As expected, patients in the earlier two time windows were more likely to have been treated with IV tPA before mechanical thrombectomy. Importantly, there was no difference in time from hospital arrival to groin puncture between the groups. Patients who achieved successful reperfusion earlier arrived at the hospital earlier after symptom onset. The full distribution of OTR times for the entire cohort can be found in Figure 1.
TABLE 1.
Univariate Baseline Characteristics of Ischemic Stroke Patients in Onset-to-Reperfusion Times Groups
| Patient Characteristic | All Patients n = 202 |
Group 1 (124–240 min) n = 54 |
Group 2 (241–360 min) n = 99 |
Group 3 (361–660 min) n = 49 |
pa |
|---|---|---|---|---|---|
| Age, mean (SD) | 68 (13) | 68 (12) | 68 (13) | 69 (12) | 0.895 |
| Female (%) | 62 | 63 | 67 | 53 | 0.273 |
| NIHSS, median (IQR) | 17 (14–20) | 15 (11–19) | 17 (15–20) | 18 (13–20) | 0.043 |
| NIHSS, categorical, % | 0.136 | ||||
| 8–12 | 20 | 31 | 15 | 20 | |
| 13–17 | 33 | 29 | 36 | 29 | |
| 18–22 | 36 | 33 | 33 | 43 | |
| 23–42 | 11 | 6 | 16 | 8 | |
| Medical history, % | |||||
| Atrial fibrillation | 38 | 35 | 37 | 41 | 0.839 |
| Hypertension | 61 | 59 | 60 | 67 | 0.615 |
| Coronary artery disease/myocardial disease |
23 | 28 | 19 | 24 | 0.455 |
| Diabetes | 18 | 11 | 14 | 33 | 0.007 |
| Hyperlipidemia | 39 | 50 | 37 | 31 | 0.116 |
| Peripheral artery disease | 1 | 0 | 2 | 2 | 0.574 |
| Smoking | 15 | 13 | 16 | 16 | 0.851 |
| Previous stroke/TIA | 18 | 20 | 19 | 14 | 0.692 |
| Prestroke modified Rankin Scale, mean (SD) |
0.4 (0.8) | 0.4 (0.7) | 0.4 (0.7) | 0.5 (0.9) | 0.524 |
| Prestroke modified Rankin Scale, categorical, % |
0.422 | ||||
| 0 | 71 | 74 | 70 | 67 | |
| 1 | 16 | 17 | 15 | 17 | |
| 2 | 12 | 6 | 15 | 12 | |
| 3 | 2 | 2 | 0 | 5 | |
| Target occlusion location, % | 0.093 | ||||
| ICA | 13 | 13 | 14 | 12 | |
| M1 | 71 | 70 | 76 | 61 | |
| M2 | 15 | 17 | 10 | 22 | |
| M3 | 1 | 0 | 0 | 4 | |
| Baseline ASPECTS mean (SD) |
8.3 (1.7) | 8.4 (1.3) | 8.2 (1.7) | 8.2 (1.9) | 0.857 |
| Receipt of IV t-PA, % | 55 | 52 | 64 | 43 | 0.078 |
| Baseline serum glucose, mean (SD) |
127 (59) | 110 (48) | 135 (71) | 130 (40) | 0.056 |
| Number of Solitaire passes, mean (SD) |
1.5 (0.7) | 1.4 (0.7) | 1.5 (0.7) | 1.7 (0.9) | 0.183 |
| Time from onset to hospital arrival, median (IQR) |
175 (70–245) | 61 (45–94) | 180 (93–225) | 300 (248–345) | <0.001 |
| Time from hospital arrival to groin puncture, median (IQR) |
82 (53–126) | 83 (55–111) | 73 (48–141) | 84 (56–121) | 0.561 |
| Time from onset to groin puncture, median (IQR) |
255 (197–315) | 160 (123–180) | 257 (236–281) | 382 (336–426) | <0.001 |
| Time from onset to reperfusion, median (IQR) |
300 (236–360) | 198 (170–217) | 302 (272–330) | 432 (390–478) | <0.001 |
| Time from onset to reperfusion, mean (SD) |
305 (97) | 194 (31) | 300 (34) | 438 (61) | <0.001 |
p values for continuous variables calculated by analysis of variance between the groups (when mean is reported) or Kruskal-Wallis test between the groups (when median is reported); p values for discrete variables calculated by chi-squared test.
SD = standard deviation; NIHSS = National Institutes of Health Stroke Scale/Score; IQR = interquartile range; TIA = transient ischemic attack; ICA = internal carotid artery; IV = intravenous; tPA = tissue-type plasminogen activator,
FIGURE 1.
Histogram of onset to reperfusion times for the entire cohort of patients studied in this analysis (n = 202). [Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
Independent demographic and presenting clinical features associated with differences in OTR are shown in Table 2. In multivariate linear regression analysis, each 5-point increase in NIHSS was associated with a 16-minute increase in OTR. Female sex and history of hyperlipidemia were associated with reduced OTR of 26 and 29 minutes. A history of diabetes was associated with a significant delay in OTR of 49 minutes.
TABLE 2.
Patient and Treatment Characteristics Independently Associated with Earlier Onset-to-Reperfusion Times
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Patient Characteristic | Estimate | SE | p | Estimate | SE | p |
| Age, per 5-year increments | 1.92 | 2.73 | 0.483 | — | — | — |
| Female sex | −20.4 | 14.01 | 0.146 | −25.8 | 14.26 | 0.072 |
| NIHSS, per 5-point increments | 13.41 | 7.56 | 0.078 | 15.83 | 7.33 | 0.032 |
| Atrial fibrillation | 19.74 | 14.02 | 0.161 | — | — | — |
| Hypertension | 5.32 | 14.01 | 0.704 | — | — | — |
| Coronary artery disease/ myocardial disease |
−14.8 | 16.24 | 0.362 | — | — | — |
| Diabetes | 70.65 | 17.12 | 0.000 | 49.17 | 17.98 | 0.007 |
| Hyperlipidemia | −31.0 | 13.81 | 0.026 | −28.8 | 13.96 | 0.040 |
| Peripheral artery disease | 39.76 | 56.35 | 0.481 | — | — | — |
| Smoking | 6.37 | 18.93 | 0.737 | — | — | — |
| Previous stroke/TIA | −13.1 | 17.62 | 0.458 | — | — | — |
| mRS 1—5 | 12.46 | 16.09 | 0.440 | — | — | — |
| M1 vs. ICA | −7.75 | 19.41 | 0.690 | — | — | — |
| M2 vs. ICA | 17.13 | 27.75 | 0.540 | — | — | — |
| M3 vs. ICA | 78.00 | 61.09 | 0.213 | — | — | — |
| Receipt of IV t-PA | −23.2 | 14.27 | 0.106 | — | — | — |
| Baseline ASPECTS ≥ 6 | −25.2 | 22.38 | 0.261 | — | — | — |
NIHSS = National Institutes of Health Stroke Scale/Score; TIA = transient ischemic attack; mRS = modified Rankin Scale; ICA = internal carotid artery; IV = intravenous; tPA = tissue-type plasminogen activator; SE = standard error.
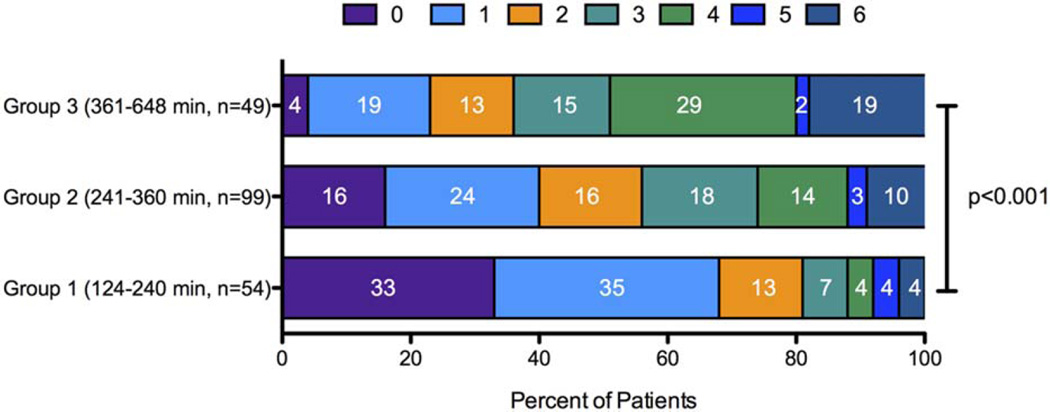
The unadjusted association of clinical outcomes with onset to reperfusion times are shown in Table 3 and Figure 2. Functional outcomes were best with treatment in the 2- to 4-hour window, intermediate with treatment in the 4- to 6-hour window, and least good with treatment in the 6- to 11-hour window. These findings were very similar in the subsets of patients who did not have diabetes (p < 0.05) and those treated with a single Solitaire pass (p < 0.01). Patients who achieved OTR in the 120- to 240-minute window had the lowest levels of disability at 90 days. For this group, mean mRS was 1.4, with 69% achieving mRS 0 to 1 and 81% achieving 0 to 2. There were no OTR group differences in rates of symptomatic intracranial hemorrhage, which were low, nor length of hospitalization. Nominal increases in mortality with later treatment did not reach statistical significance.
TABLE 3.
Univariate Outcomes Associated With Onset-to-Reperfusion Groups
| Outcome | All Patients n = 202 |
Group 1 (124–240 min) n = 54 |
Group 2 (241–360 min) n = 99 |
Group 3 (361–660 min) n = 49 |
p-valuea |
|---|---|---|---|---|---|
| 90 day modified Rankin scale, mean (SD) |
2.3 (1.9) | 1.4 (1.6) | 2.4 (1.8) | 3.3 (1.8) | <.001 |
| 90 day modified Rankin scale, median (IQR) |
2.3 (1.9) | 1.4 (1.6) | 2.4 (1.8) | 3.3 (1.8) | <.001 |
| mRS 0–1, % | 43 | 69 | 40 | 23 | <.001 |
| mRS 0–2, % | 58 | 81 | 55 | 35 | <.001 |
| mRS 0–3, % | 72 | 89 | 73 | 50 | <.001 |
| Symptomatic intracerebral hemorrhage, % |
3 | 2 | 4 | 4 | 0.751 |
| PH-2 intracerebral hemorrhage, % |
1 | 0 | 2 | 2 | 0.574 |
| Any intracerebral hemorrhage, % |
15 | 9 | 17 | 16 | 0.398 |
| In-hospital mortality, % | 5 | 2 | 6 | 8 | 0.345 |
| Length of hospitalization, median (IQR) |
7.7 (5.1) | 7.6 (3.9) | 7.6 (5.8) | 8.2 (5.0) | 0.721 |
p values for continuous variables calculated by analysis of variance (when mean is reported) or Kruskal-Wallis test (when median is reported); p values for discrete variables calculated by chi-squared test.
SD = standard deviation; IQR = interquartile range; mRS = modified Rankin Scale.
FIGURE 2.
Ninety-day mRS outcomes divided by onset-to-reperfusion groups. Stacked bar graphs demonstrate full mRS outcome distributions for the examined cohort divided into three groups based on time of onset to reperfusion. Numbers within each colored region represent the percentage of patients with the corresponding mRS outcome grade for that group (p < 0.001, chi-squared test). mRS = modified Rankin Scale. [Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
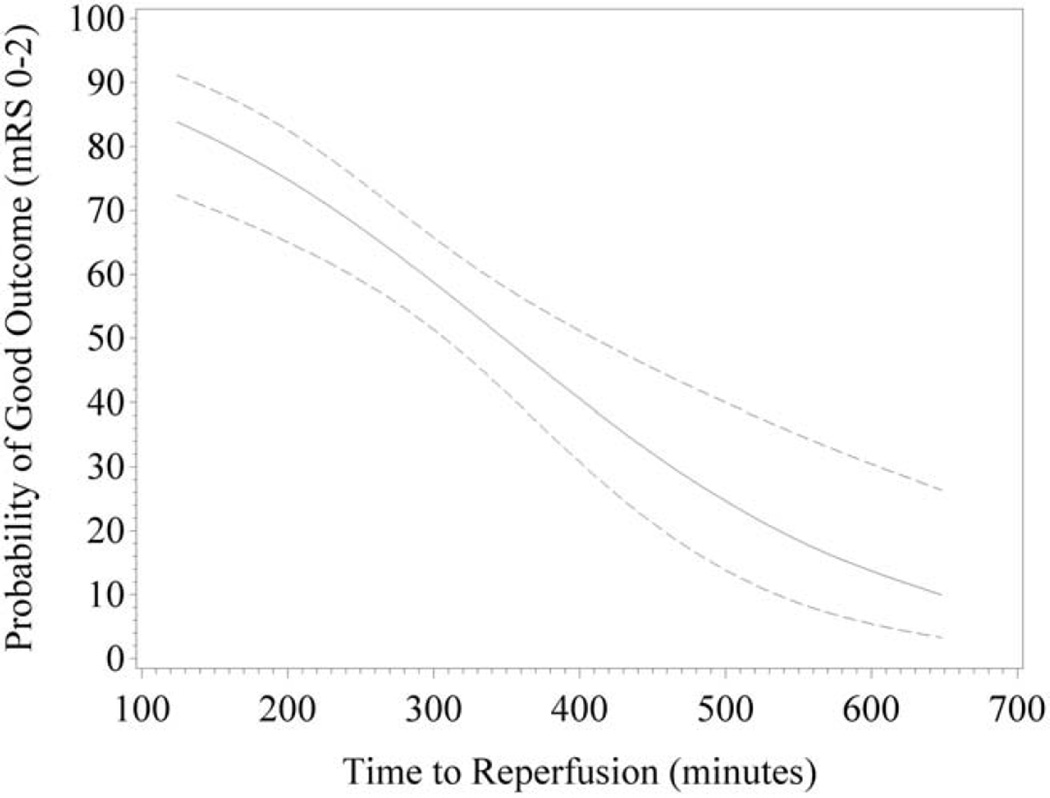
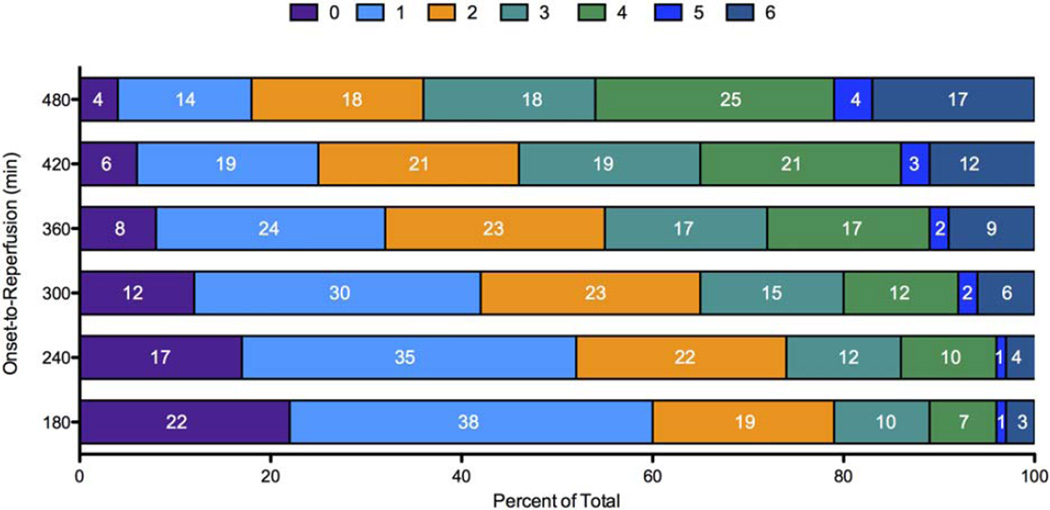
The independent effect of OTR on the binary endpoint of nondisabled outcome (mRS 0–2) at 90 days is shown in Figure 3. The likelihood of a good clinical outcome was more than 80% for patients who achieved OTR of 120 minutes, approximately 50% for patients with an OTR of 360 minutes, and less that 20% for patients with an OTR of 600 minutes. The independent effect of OTR on the ordinal distribution of mRS outcomes, after adjustment for baseline characteristics, is shown in Figure 4. The c-statistic for the predictive model was 0.757. Reductions in disability across the entire mRS outcome range were demonstrated in every 60-minute interval from 180 to 480 minutes OTR.
FIGURE 3.
Predicted probability and confidence interval of good neurological outcome (mRS 0–2) at 90 days from logistic regression with time as a continuous variable. Probability of mRS 0 to 2 is plotted against onset to recanalization. Dashed lines demonstrate 95% confidence intervals. mRS = modified Rankin Scale.
FIGURE 4.
Predicted 90-day mRS outcomes from adjusted ordinal logistic regression. Stacked bar graphs represent the predicted mRS outcome distributions for each incremental 60-minute change in onset-to-reperfusion time, beginning with 180 minutes. Numbers within each colored region represent the percentage of patients with the corresponding mRS outcome grade for that time window. mRS = modified Rankin Scale. [Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
The benefit of faster reperfusion was substantial. In the primary algorithmic joint outcome table analysis, considering transitions over all 7 levels of the mRS, the number needed to treat was 30 for one more patient to benefit from 15-minute faster achievement of substantial reperfusion. Among 1,000 patients treated 15 minutes faster, 34 would have a less disabled outcome. Conversely, for every 5-minute delay in substantial endovascular reperfusion, 1 of every 100 treated patients has a worse disability outcome. In the sensitivity analyses, the permutation test and bootstrapped automated random sampling methods yielded identical results, with NNT of 28 for both. Table 4 shows NNT and BPT values for faster reperfusion, comparing ordinal and dichotomized analyses of the mRS. Ordinal analysis revealed a more profound effect of OTR on ultimate clinical outcome. With ordinal analysis, the BPT for 15-minute faster OTR was 1.5- to 4.7-fold higher than observed with dichotomized analysis.
TABLE 4.
Benefit Associated With Every 15-Minute Acceleration of Substantial Reperfusion
| Number Needed to Treat |
Benefit Per Thousand Treated |
|
|---|---|---|
| For transitions across multiple mRS levels | ||
| All seven levels (0, 1, 2, 3, 4, 5, 6) | 30 | 34 |
| Best six levels (0, 1, 2, 3, 4, 5/6) | 30 | 34 |
| For individual dichotomizations of the mRS | ||
| 0 vs. 1–6 | 104 | 10 |
| 0–1 vs. 2–6 | 48 | 21 |
| 0–2 vs. 3–6 | 46 | 22 |
| 0–3 vs. 4–6 | 58 | 17 |
| 0–4 vs. 5–6 | 115 | 9 |
| 0–5 vs. 6 | 140 | 7 |
mRS = modified Rankin Scale.
Discussion
In this study of over 200 patients treated with the Solitaire stent-retriever device in the combined SWIFT and STAR trial cohorts who achieved successful reperfusion, we found that accelerated onset to reperfusion was associated with improved disability outcomes. Compared to binary assessments, ordinal analysis showed an average 3-fold greater effect in improving clinical outcomes. For every 1,000 patients treated, every 15-minute acceleration in OTR was associated with 34 more patients having reduced final disability by one or more levels on the modified Rankin scale. Very early reperfusion, within 120 minutes from symptom onset, was associated with a greater than 80% chance of a nondisabled final (mRS 0–2) 3-month outcome.
We found that analyses that incorporate the full range of disability as measured by the mRS demonstrate a profound effect of OTR on ultimate clinical outcome. We detected real improvements in outcome with as brief as 15-minute increments in OTR. The overall effect of OTR on clinical outcome was 3-fold greater than that as determined with simple binary analysis. Ordinal benefit per thousand values differed mildly when derived using the automated algorithmic joint outcome table method (primary analysis) and the permutation tests (sensitivity analyses). This finding is consistent with the known proneness of the permutation test to attenuate correlations toward the null value when nondifferential misclassification may occur, as can arise with the mRS.19
Our findings may usefully be contrasted with previous studies. Though we are unaware of previous studies that have examined the ordinal effect of OTR for ET, there has been a previous analysis of ordinal impact for faster onset to treatment time with intravenous tPA.20 Our study found a nearly 2-fold greater benefit of 15 minutes faster OTR with ET than 15 minutes faster onset to treatment time with intravenous tPA. This difference is likely owing to reperfusion rates. The current analysis was confined to patients in whom substantial reperfusion was achieved. In the intravenous tPA treatment analysis, only 40% to 50% of treated patients would have achieved early reperfusion as a result and benefitted from faster therapy.
Our study confirms and extends previous studies of the effect of ET OTR, which have focused on dichotomized outcomes.9,10,21 The high proportion of nondisabled outcomes in early reperfusion time frames in the present study is particularly notable in comparison to previous studies of ET. In this cohort, we found that 81% of patients with OTR in the earliest time window (120–240 minutes) achieved mRS 0 to 2 at 90 days. This finding is in spite of presenting with moderate-to-severe strokes, with NIHSS on presentation of 15 (IQR, 11–19). This frequency of nondisabled outcome percentage is substantially higher, compared to the 52% observed in patients in the IMS III trial with TICI 2b/3 reperfusion in less than 300 minutes.21 The difference between these two numbers, in part, reflects the earlier time window of analysis in the present study (upper limit of 240 rather than 300 minutes), but this difference is also maintained throughout the studied time intervals.
In continued comparison of the two curves, the curve generated by the current cohort demonstrates the continued influence of time to recanalization on clinical outcomes over a longer time scale, up to 10 hours. In addition, the overall rate of mRS 0 to 2 at 90 days is higher, at both the early time points (350 minutes, approximately 50% vs. 35%, SWIFT-STAR vs. IMS III) and as well as the later time points (400 minutes, approximately 42% vs. 20%, SWIFT-STAR vs. IMS III). Thus, the present curve demonstrates greater absolute values and a slower decline over time. These differences between studies may be owing to advancements in modern-day ET techniques and devices. Stent-retriever therapy is associated with substantially lower rates of intracranial hemorrhage complications than previous endovascular reperfusion techniques, so that the benefits of early as well as late reperfusion are less often mitigated by an accompanying hemorrhagic adverse event.14,22
It is also worth noting that the benefit of rapid revascularization that we identify appears to be independent of baseline ASPECTS. In our study, patients treated in the early, intermediate, and late time intervals all had similar pretreatment ASPECTS but patients presenting in the latest time periods had worse outcomes. This finding may be explained by the insensitivity of noncontrast brain CT to evolving ischemia in the very early time period. The patients who presented later had higher NIHSS and were more likely to be diabetic, which suggests the presence of more advanced ischemic injury that was not captured on CT, but could lead to worsened outcomes.
Our findings once again highlight the importance of accelerating OTR. Several researchers have proposed improved workflows to streamline care from the earliest prehospital setting, to preprocedural hospital care, and through to intraprocedural techniques.23,24 Features associated with more rapid OTR have included routing patients to high volume stroke centers, such as comprehensive stroke centers (CSCs).25 Implementing prehospital stroke severity scales have been successful identifying patients with large artery occlusions and may be used as a screening tool to route patients to these centers.26 A drawback of such “direct-to-CSC” approaches, however, is the possible delay in administration of intravenous tPA, if a primary stroke center were bypassed as a result. Though the benefit in clinical outcomes with earlier intravenous tPA delivery for equivalent time periods has been shown to be less than what we identify in this study of ET, it should be noted that studies on the effect of intravenous tPA include much broader cohorts (large and small vessel strokes) and do not routinely assess recanalization, only medication administration.20 As such, although the findings from our study suggest potential benefit for a direct-to-CSC approach when the additional travel time would be small, this finding needs to be confirmed by controlled, clinical trials.
Our study has limitations. Our study used only automated techniques to derive treatment impact values in ordinal analysis. As a result, we could only derive net benefit per thousand patients. Expert population of joint outcome tables would allow disambiguated benefit and harm values per thousand patients to be derived, but would require subjective judgments by content experts.
In this study, we find that ordinal analyses of outcomes detect a far greater effect on patient outcome of early reperfusion post-ET in AIS. Specifically, we find that for every 5-minute delay in substantial endovascular reperfusion, 1 of every 100 patients treated has a worse disability outcome. As clinicians, we are constantly reminded of the challenges brought on by neurological deficits in our patients. More granular analyses such as these that are sensitive to detect smaller changes in disability allow for richer discussions when advising on treatment approaches, as well as providing counseling in the acute setting.
Acknowledgments
We thank Drs Jill Schafer and Jeffrey Gornbein for their assistance with the statistical analyses presented in this article.
Potential Conflicts of Interest
Drs. Saver and Jahan have served as an unpaid site investigator in multicenter trials run by Lundbeck and Covidien for which the UC Regents received payments on the basis of clinical trial contracts for the number of subjects enrolled. The University of California has patent rights in retrieval devices for stroke. The University of California receives funding for Dr Saver’s services as a scientific consultant regarding trial design and conduct to Medtronic/Covidien, Stryker, Neuravi, and Boehringer Ingelheim (prevention only). Dr. Saver serves as an unpaid consultant to Genentech advising on the design and conduct of the PRISMS trial; neither the University of California nor Dr. Saver received any payments for this voluntary service. Drs Levy, Gralla, Nogueira, Zaidat, and Pereira have served as site investigators and/or consultants for Coviden. Dr. Levy served as a principal investigator for the Covidien USA SWIFT PRIME Trial.
Footnotes
Authorship
Sunil A. Sheth was responsible for substantial contributions to the conception and design of the work, drafting and critically revising it and its final approval. Jeffrey L. Saver was responsible for the conception of the work, revising the article, and for its final approval. Reza Jahan, Jan Gralla, Vitor M. Pereira, Raul G. Nogueira, Elad I. Levy and Osama O. Zaidat were responsible for data acquisition, revising the article and for its final approval. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, et al. EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, et al. SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL. Time is brain—quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 6.Lees KR, Bluhmki E, Kummer von R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 7.Lansberg MG, Schrooten M, Bluhmki E, et al. Treatment time-specific number needed to treat estimates for tissue plasminogen activator therapy in acute stroke based on shifts over the entire range of the modified Rankin Scale. Stroke. 2009;40:2079–2084. doi: 10.1161/STROKEAHA.108.540708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khatri P, Abruzzo T, Yeatts SD, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazighi M, Chaudhry SA, Ribo M, et al. Impact of onset-to-reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation. 2013;127:1980–1985. doi: 10.1161/CIRCULATIONAHA.112.000311. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira RG, Smith WS, Sung G, et al. Effect of time to reperfusion on clinical outcome of anterior circulation strokes treated with thrombectomy: pooled analysis of the MERCI and Multi MERCI trials. Stroke. 2011;42:3144–3149. doi: 10.1161/STROKEAHA.111.624163. [DOI] [PubMed] [Google Scholar]
- 11.Saver JL. Optimal end points for acute stroke therapy trials: best ways to measure treatment effects of drugs and devices. Stroke. 2011;42:2356–2362. doi: 10.1161/STROKEAHA.111.619122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol. 2004;61:1066–1070. doi: 10.1001/archneur.61.7.1066. [DOI] [PubMed] [Google Scholar]
- 13.Bath PMW, Lees KR, Schellinger PD, et al. Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012;43:1171–1178. doi: 10.1161/STROKEAHA.111.641456. [DOI] [PubMed] [Google Scholar]
- 14.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 15.Pereira VM, Gralla J, Davalos A, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using solitaire flow restoration in acute ischemic stroke. Stroke. 2013;44:2802–2807. doi: 10.1161/STROKEAHA.113.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 17.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 18.Saver JL, Gornbein J, Grotta J, et al. Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3- to 4.5-hour window: joint outcome table analysis of the ECASS 3 trial. Stroke. 2009;40:2433–2437. doi: 10.1161/STROKEAHA.108.543561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard G, Waller JL, Voeks JH, et al. A simple, assumption-free, and clinically interpretable approach for analysis of modified Rankin outcomes. Stroke. 2012;43:664–669. doi: 10.1161/STROKEAHA.111.632935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 21.Khatri P, Yeatts SD, Mazighi M, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014;13:567–574. doi: 10.1016/S1474-4422(14)70066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun CH, Nogueira RG, Glenn BA, et al. “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013;127:1139–1148. doi: 10.1161/CIRCULATIONAHA.112.000506. [DOI] [PubMed] [Google Scholar]
- 24.Menon BK, Almekhlafi MA, Pereira VM, et al. Optimal workflow and process-based performance measures for endovascular therapy in acute ischemic stroke: analysis of the Solitaire FR thrombectomy for acute revascularization study. Stroke. 2014;45:2024–2029. doi: 10.1161/STROKEAHA.114.005050. [DOI] [PubMed] [Google Scholar]
- 25.Gupta R, Horev A, Nguyen T, et al. Higher volume endovascular stroke centers have faster times to treatment, higher reperfusion rates and higher rates of good clinical outcomes. J Neurointerv Surg. 2013;5:294–297. doi: 10.1136/neurintsurg-2011-010245. [DOI] [PubMed] [Google Scholar]
- 26.Nazliel B, Starkman S, Liebeskind DS, et al. A brief prehospital stroke severity scale identifies ischemic stroke patients harboring persisting large arterial occlusions. Stroke. 2008;39:2264–2267. doi: 10.1161/STROKEAHA.107.508127. [DOI] [PMC free article] [PubMed] [Google Scholar]