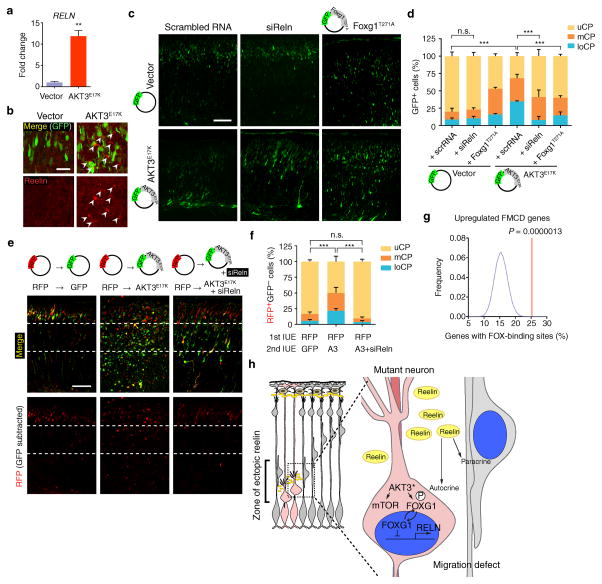
Figure 6. Neuronal migration defects rescued by Reln siRNA.
(a) Quantitative real-time PCR was performed to measure RELN expression in hNPCs expressing AKT3E17K and compared to vector-expressing hNPCs. Three independent experiments were quantified in triplicate. (b) Mouse embryos at E14.5 were electroporated with indicated DNA constructs. Brains isolated at E18.5 used for reelin expression. Arrowheads: reelin immunostaining in pericellular area of ectopic neurons (intensity-per-pixel: 622.55 ± 45.80 compared to 4092.77 ± 9.46, marginal zone). (c) E14.5 embryos were co-electroporated with indicated constructs or RNAs. (d) Localization of GFP+ neurons at E18.5 was quantified in each cortical region (n = 3 for each). (e, f) Developing cortices were in utero electroporated (IUE) at E14.5 with RFP vector. After 15 min, embryos were sequentially electroporated with either GFP vector (n = 3) or GFP also expressing AKT3E17K (A3) with or without Reln siRNA (n = 6 and 4, respectively). At E18.5, brains were sectioned for imaging. Localization of RFP+GFP− cells was quantified in each cortical region. Values: mean ± s.d. n.s., not significant; **, P < 0.01; ***, P < 0.001, Student’s t-test (a); G-test of goodness-of-fit (d, f). Scale bars: 25 μm (b); 100 μm (c, e). (g) Enrichment of FOX-binding site. See Online Methods for details. (h) Model of effect of AKT3 mutation on surrounding cells. Mutant cell, pink; surrounding cells, gray. AKT3* activating somatic mutation leads to mTOR activation and phosphorylation and cytoplasmic sequestration of FOXG1, relieving repression of RELN, which is then secreted by mutant cells to act in an autocrine (cell autonomous) and paracrine (non-cell autonomous) fashion.