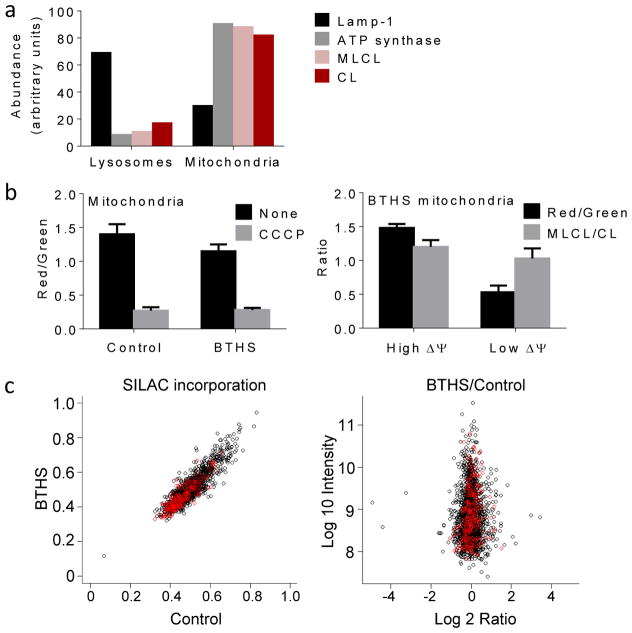
Figure 3. MLCL is present in intact mitochondria with normal protein turnover.
(a) Lysosomes and mitochondria were prepared from BTHS lymphoblasts. Marker proteins for lysosomes (Lamp-1) and mitochondria (α-subunit of ATP synthase) were quantified by Western blotting; MLCL and CL were quantified by MS. CL and MLCL were associated with mitochondria. Data are means of duplicate determinations. (b) Mitochondria were isolated from human lymphoblasts and analyzed by flow cytometry after staining with JC-1. The membrane potential, measured by the red/green fluorescence ratio, was slightly lower in BTHS mitochondria than in controls (P<0.03, t-test). CCCP collapsed the membrane potential. Isolated BTHS mitochondria were divided with a cell sorter into two populations one with high and one with low membrane potential (ΔΨ). The populations were analyzed by flow cytometry and MS, which confirmed the difference in membrane potential but did not show any difference in the MLCL/CL ratio. Data are means±s.e.m. (N=3). (c) Human lymphoblasts were cultured with stable isotope labeled amino acids. The level of incorporation of labeled amino acids into mitochondrial (red circles) and non-mitochondrial (black circles) proteins was measured by proteomic analysis. The BTHS/control ratio of incorporated label was measured in 1:1 mixtures of control and BTHS samples, each labeled with different precursors. The turnover of mitochondrial proteins was similar in BTHS and controls.