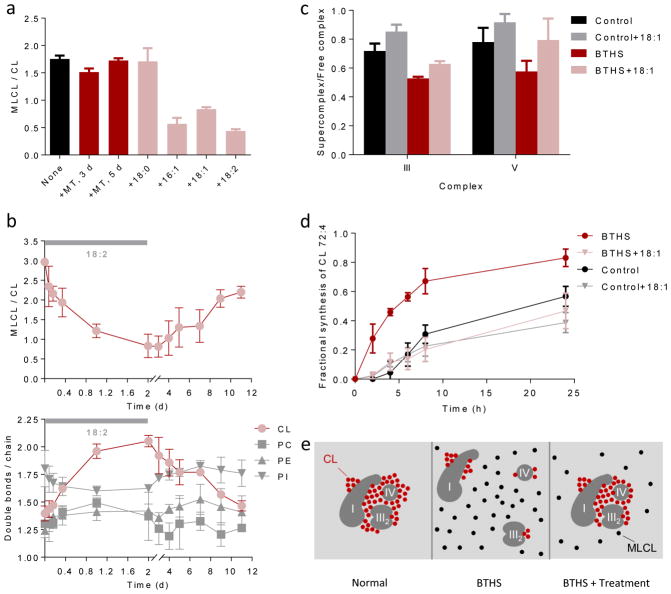
Figure 6. Unsaturated fatty acids stabilize CL.
(a) BTHS lymphoblasts were cultured with 5 μM MitoTempo (MT) for 3 or 5 days or with 0.1 mM exogenous fatty acid (stearic acid, 18:0; palmitoleic acid, 16:1; oleic acid, 18:1; linoleic acid, 18:2) for 4 days. Unsaturated fatty acids decreased the MLCL/CL ratio (P<0.003, t-test). (b) BTHS lymphoblasts were cultured first with and then without linoleic acid. The MLCL/CL ratio inversely correlated with the CL unsaturation. (c–d) Lymphoblasts were cultured ± 18:1 for 4 days followed by measurements of supercomplex abundance and CL turnover with 2H33-oleic acid. BTHS reduced the proportion of supercomplexes and increased the turnover of CL (P<0.03, t-test). 18:1 increased the proportion of supercomplexes and reduced the turnover of CL in BTHS but not in controls (P<0.02, t-test). Half-lives of CL 72:4, estimated by non-linear regression analysis, were 20 hours (control), 30 hours (control+18:1), 5 hours (BTHS), and 26 hours (BTHS+18:1). Data in panels a–d are means±s.e.m. (N=3). (e) The cartoon shows supercomplex I1-III2-IV1 as an example; a similar mechanism is suggested for other complexes. In normal mitochondria, unsaturated CL clusters around protein complexes, which promotes their assembly to supercomplexes and protects CL from degradation. In BTHS, the fatty acid composition of CL is altered. Less CL is associated with proteins and non-associated CL is hydrolyzed to MLCL. Treatments that increase CL unsaturation or force the formation of supercomplexes, increase the proportion of protein-associated CL. As a result, less CL is hydrolyzed to MLCL.