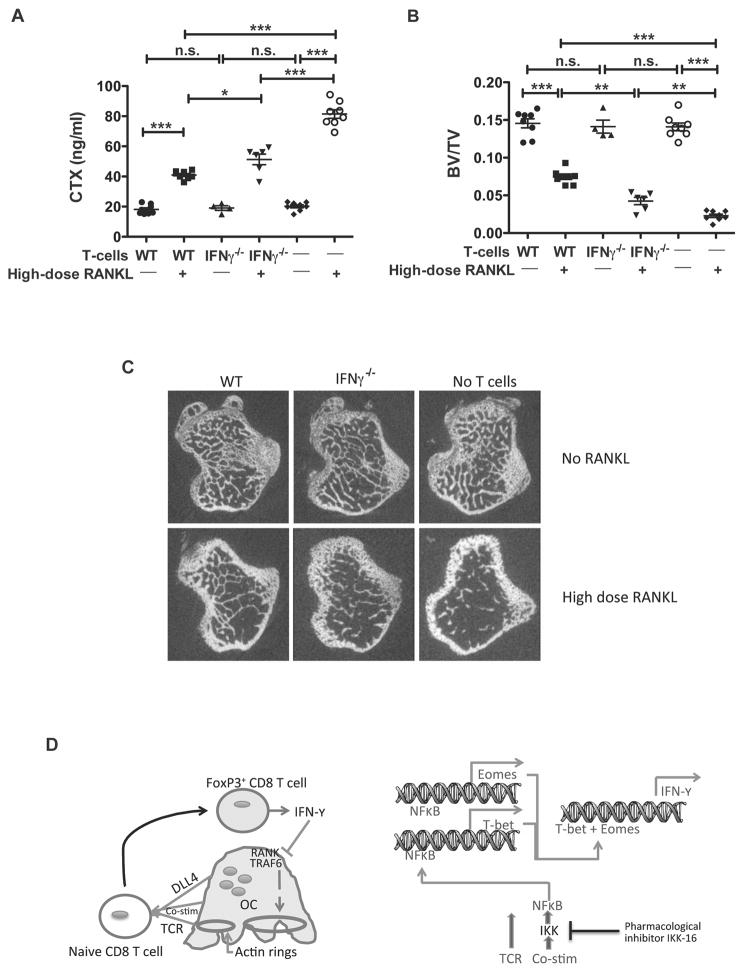
Figure 5. IFN-γ is important for the bone protective effect of TcREG in vivo.
(A) WT or IFN-γ−/− CD8 T-cells were adoptively transferred into OT-II mice (n=4–8 mice/group) followed 24 h later by administration of 1 mg/kg of RANKL daily for 2 days. Serum CTX levels at 50 h after first RANKL injections are shown to assess relative bone resorption levels. IFN-γ−/− CD8 T-cells have reduced ability to suppress RANKL-induced bone resorption as compared to WT CD8 T-cells. (B) μCT of proximal tibia of mice from the experiment described in (A). Reconstitution of OT-II mice with IFN-γ−/− CD8 T-cells partially protected mice, relative to WT T-cells, from losing bone volume in response to 1 mg/kg of RANKL resulting in intermediate levels of BV/TV. Consistent with these results, measurement of bone mineral density gave corresponding results. * p<0.05, ** p<0.01, *** p< 0.001. (C) Representative μCT images from the experiment described in (B). (D) A model summarizing the feedback loop between osteoclasts and CD8 T-cells: Left panel: Osteoclast are antigen-presenting cells that provide T cell receptor (TCR) activation, co-stimulation, and differentiation (Delta like ligand 4 [DLL4]) signals to naïve CD8 T-cells. This leads to induction of FoxP3 and IFN-γ in the CD8 T-cell. IFN-γ in turn causes degradation of TRAF6 and suppression of actin ring formation in osteoclasts inhibiting bone resorption. Right panel: A proposed model of events triggered in CD8 T-cells. Upon interaction with osteoclasts, NF-κB signaling is activated in the T-cells, leading to expression of transcription factors T-bet and Eomes, which then activate transcription of IFN-γ.