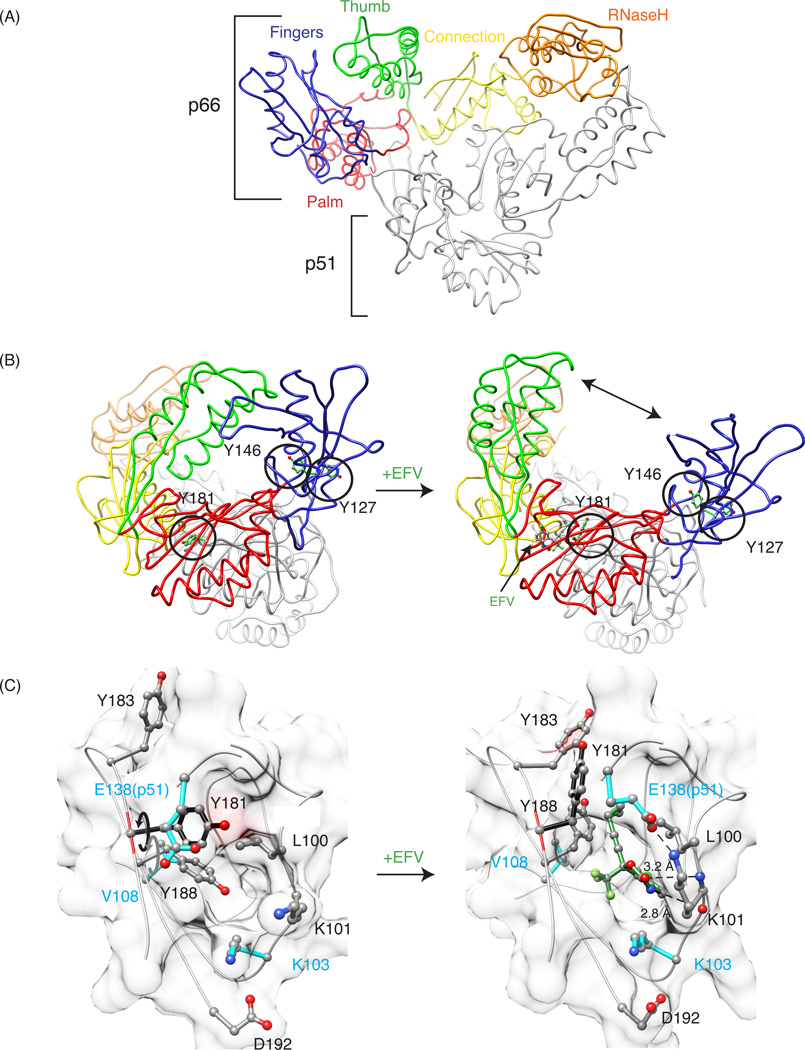
Figure 1.
General description of RT structure, and comparison of apo and EFV-bound crystal structures of RT. (A) Tube representation of apo-RT (PDB: 1DLO), with the fingers, palm, thumb, connection, and RNase H domains in the p66 subunit colored in blue, pink, green, yellow and orange, respectively. The p51 subunit is colored grey. (B) Structural differences between apo-RT (left, PDB: 1DLO) and EFV-bound RT (right, PDB: 1FK9). A large conformational change, including the separation of the thumb and fingers domains (indicated by the arrow), is seen in the drug-bound structure. Tyrosine residues 127, 146 and 181 are depicted in ball and stick representation and encircled. (C) Details of the binding site in apo RT and the EFV-bound RT complex, illustrating the rotation of the Y181 (black arrow) and Y188 (grey arrow) side chains out of the binding pocket. The bound EFV molecule is shown in green and pertinent distances between the benzoxazin-2-one and the backbone carbonyl oxygen of K101 (2.8 Å), and the carbonyl group of the bexzoxazine-2-one and the backbone nitrogen of atom K101 (3.2 Å) are indicated.