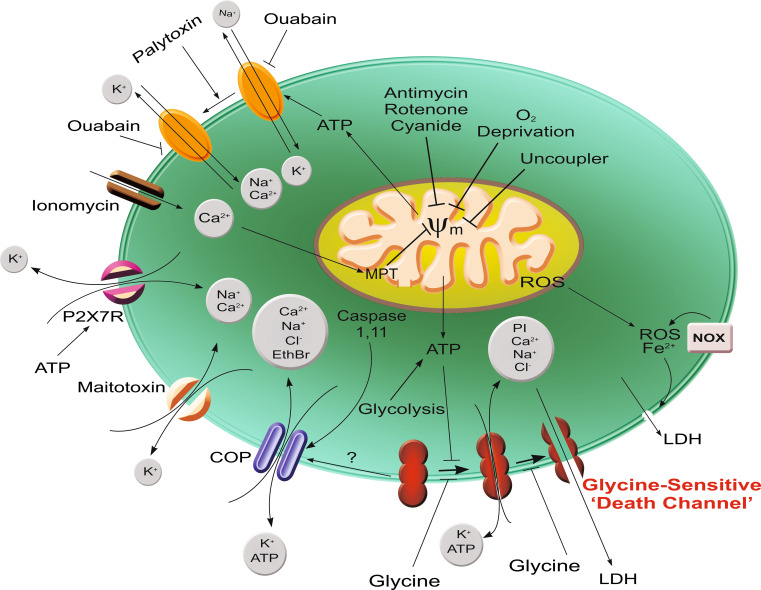
Fig. 1.
Pathways for development of glycine-sensitive cell death. Summarized here are pathways of injury discussed in the text for which glycine protection is well documented and a scheme for ‘death channel’ development that incorporates data from studies with differently sized fluorescent probes. Primary insults include multiple maneuvers that impair mitochondrial ATP generation (oxygen deprivation, electron transport inhibitors, uncouplers, Ca2+-induced development of the mitochondrial permeability transition), maneuvers that produce transmembrane cation shifts prominently including increased Ca2+ entry (ionomycin, maitotoxin, palytoxin-induced modification of Na, K, ATPase, and P2X7 receptor activation), and activation of caspase 1 and/or 11. The glycine-insensitive ‘cytolytic oncotic pore’ mediating ethidium bromide uptake, which develops after P2X7 receptor activation, maitotoxin and likely caspase activation promotes the cation shifts and can also lead to loss of ATP. This further enhances the ATP depletion. Development of the glycine-sensitive ‘death channel’ is depicted as a sequential process of pore enlargement that is normally suppressed by ATP, because ATP depletion is a common factor in most processes and other major injury mediators such as Ca2+ increases are not necessary for it to occur. It is possible that the cytolytic oncotic pore rather than being a separate process as depicted is a stage in the development of the glycine-sensitive channel. Cell death associated with ROS and Fe2+-mediated lipid peroxidation directly targeting the lipid phase of the membrane is shown as a separate pathway, since it is relatively glycine insensitive. Other early injury-associated membrane permeability changes such as activation of connexin [268] and TRPM [103] channels almost certainly feed into the pathways shown, but are not illustrated because they have not been specifically studied with glycine