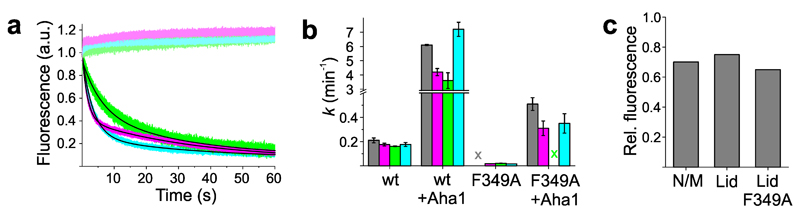
Figure 3. Influence of Aha1 on kinetics of local motions.
(a) AMP-PNP-triggered fluorescence intensity time traces of β-strand swap (A2C+E162W, magenta), lid closure (S51C-A110W, green), and N/M-domain association (E192C-N298W, cyan). Samples were incubated with Aha1 before measurement and time traces were recorded using stopped-flow spectroscopy. Data in shaded color are control samples that lacked the engineered Trp. Fluorescence transients were fitted using a bi-exponential model including a linear baseline drift of minor amplitude (black line). (b) Rate constants of ATP hydrolysis (gray) by wild-type Hsp90 (wt) and mutant F349A together with the corresponding mean rate constants of β-strand swap (magenta), lid closure (green), and N/M-domain association (cyan) measured in absence and presence of Aha1 (X = no kinetics detectable). Data represent mean values ± s.d. of three measurements. (c) Equilibrium fluorescence intensities measured from reporters of N/M-domain association and of the lid on wild-type Hsp90 and mutant F349A after incubation with Aha1.