Abstract
PURPOSE
We aimed to evaluate the imaging features of computed tomography (CT) and angiography and the efficacy of transcatheter arterial embolization (TAE) in patients with hemobilia of different iatrogenic causes.
METHODS
Thirty patients with hemobilia were divided into two groups according to their iatrogenic causes, i.e., group 1, 11 patients (36.7%) with transhepatic intervention and group 2, 19 patients (63.3%) with surgical procedures in the hilar area. Seventeen patients (56.7%) underwent abdominal contrast-enhanced CT before selective angiography. Polyvinyl alcohol particles, gelatin sponges, and coils were used for TAE. Data from the two groups were compared using Fisher’s exact test and the Mann-Whitney U test.
RESULTS
Contrast-enhanced CT showed a hematoma, extravasation of contrast material, and pseudoaneurysm. The bleeding source was determined by angiographic features in all patients, which were not significantly different between the two groups (P = 0.127), and pseudoaneurysm was the most common. The embolic material and number of coils used for TAE were significantly different between the two groups (P < 0.001), but the embolization was technically successful in all patients. The clinical success rate of the first embolization was 100% in group 1 vs. 84.2% in group 2. The overall clinical success rate of TAE was 100% in all patients. The complication rate was 63.6% in group 1 vs. 68.4% in group 2 (P = 1.000).
CONCLUSION
CT was useful in diagnosing hemobilia, and angiograms enabled determination of the bleeding source. Pseudoaneurysm was one of the most common angiographic features. TAE was successfully performed with different embolic materials on the basis of the iatrogenic cause and bleeding location.
Hemobilia is a potentially life-threatening cause of upper gastrointestinal hemorrhage (1, 2). Approximately 65% of all hemobilia is caused by hepatic trauma or iatrogenic injury, such as needle biopsy of the liver or hepatobiliary or pancreatic procedures (2–13). The incidence of hemobilia caused by iatrogenic injury is increasing because of the increase in hepatobiliary intervention procedures (2, 4, 13). Therefore, effective diagnosis and management of iatrogenic hemobilia are urgently required (1–13).
The management of hemobilia is directed at hemostasis and relief of biliary obstruction (3). Although associated with a high mortality rate, surgical intervention was once considered the primary method of treatment. Walter et al. (14) reported the first successful transcatheter arterial embolization (TAE) of a hepatic artery in 1976. With more recent improvements in instrumentation and embolic materials, TAE has become a safe, less invasive, and effective alternative to surgery. Because most patients do not require surgical intervention, mortality and morbidity rates have subsequently decreased (5).
The aim of this retrospective analysis was to evaluate the imaging features of computed tomography (CT) and angiography and the efficacy of and complications associated with TAE in patients with iatrogenic hemobilia.
Methods
Patients
All procedures performed in studies involving human participants were in accordance with the ethical standards of our Institutional Review Board, which waived written informed consent. From January 2005 to July 2014, 30 patients (14 men and 16 women; mean age, 47.0±13.9 years; range, 13–69 years) with iatrogenic hemobilia were included in this study.
All patients were grouped according to iatrogenic origin. Group 1 included 11 patients (36.7%) who had undergone transhepatic intervention, i.e., six cases (20%) of percutaneous transhepatic biliary drainage (PTBD), three cases (10%) of percutaneous transhepatic biopsy, and two cases (6.7%) of radiofrequency ablation; group 2 included 19 patients (63.3%) who had undergone surgical procedures in the hilar area, i.e., 13 cases (43.3%) of laparoscopic cholecystectomy for cholelithiasis and six cases (20%) of surgical resection of cholangiocarcinoma. Upper gastrointestinal endoscopy was performed in 15 cases (50%) and abdominal contrast-enhanced CT was performed in 17 cases (56.7%) (7 cases in group 1 and 10 cases in group 2) before conventional angiography.
CT examinations
Contrast-enhanced CT studies were performed with multidetector helical scanners (Philips Brilliance) with 64–256 detector rows. Images were obtained during patient breath holding using the following acquisition parameters: tube voltage, 120 kVp; automatic milliampere setting (depending on patient size), with a range of 240–360 mA; section thickness and reconstruction interval of 1.5 mm; and pitch of less than 1 (mean, 0.938±0.045). All patients received 80 mL nonionic intravenous contrast material (Omnipaque 350; Amersham Health, GE Medical Systems) using a power injector at 3.5 mL/s, and the time delays from injection of the contrast agent to scanning were approximately 25 s and 70 s for the arterial and venous phases, respectively.
Embolization technique
Digital subtraction angiography and TAE were performed by two interventional radiologists with more than eight years of experience in our conventional angiographic suite. After puncturing the right or left femoral artery, Cobra or RH 5-Fr catheters (Cook Medical) were introduced over a 0.035-inch guide wire (Terumo Corp.). Selective arteriograms of the superior mesenteric artery, celiac artery, and common hepatic artery were obtained to determine the site of bleeding. Indirect portal vein angiography before TAE was performed to ensure patency of the portal vein. After the identification of arterial injury, the specific bleeding branch vessel was selectively catheterized using 5F catheters or superselectively catheterized using a 2.6F (Asahi Intecc Co.) microcatheter. Polyvinyl alcohol (PVA, 300–500 μm, Cook Medical) particles, absorbable gelatin sponges (GS, 1×1×1 mm, Najing), coils (MWCE-35 embolization coils, Cook Medical), or microcoils (0.018-inch soft microcoil, Hilar or Tornado, Cook Medical) were then superselectively deployed to occlude the bleeding artery according to the diameter of the targeted vessel. A postembolization angiogram was obtained to confirm hemostasis and patency of the remainder of the hepatic artery and its branches.
End points for TAE
The major end points for TAE were assessments of both technical success and clinical success. Technical success was determined by the disappearance of abnormal angiographic features. Clinical success was confirmed by evaluating vital signs (blood pressure and pulse rate) and bile color and available pre-and postembolization hematocrits, without the requirement for repeat TAE or additional surgery during the hospital stay.
Complications and follow-up
Patients were examined after TAE, including blood cell counts and liver function tests. Complications associated with TAE were assessed on the basis of the Society of Interventional Radiology (SIR) clinical practice guidelines (15). Hepatic ischemia was defined as an increase in serum transaminase levels (more than three times higher than baseline) without the presence of a hypodense area on contrast-enhanced CT. Hepatic infarction was defined as an increase in serum transaminase levels (more than three times higher than baseline) with an irregular hypodense area without enhancement on contrast-enhanced CT (16). All patients were alive and showed no sign of recurrence with a mean follow-up time of 102±59 days (range, 45–350 days).
Statistical analysis
We observed the imaging findings from abdominal CT and conventional angiography and evaluated embolic material, effectiveness, and complications in two groups of patients with different iatrogenic causes. Categorical data were compared using Fisher’s exact test and the Fisher-Freeman-Halton test, and the number of coils was assessed by the Mann-Whitney U test. A P value of <0.05 was considered to be statistically significant. All analyses were performed using SPSS version 17.0 (SPSS, Inc.).
Results
Thirty patients with iatrogenic hemobilia were included in this study. The interval from iatrogenic injury to bleeding ranged 3–59 days (mean, 17.5±12.5 days). Bleeding episodes occurred between one and six times. Melena, abdominal pain, hematemesis, and jaundice were the primary symptoms. The typical clinical triad of hemobilia (melena and/or hematemesis, biliary colic, and obstructive jaundice) was observed in nine patients (30%). The diagnosis of hemobilia was confirmed by upper gastrointestinal endoscopy (a clot or hemorrhage from the papilla of Vater) and clinical symptoms in 10 patients (33.3%), by conventional angiography and clinical symptoms in three patients (10%) and by imaging features of abdominal contrast-enhanced CT, conventional angiography, and clinical symptoms in 17 patients (56.7%).
On unenhanced abdominal CT images, a hematoma was located within the hepatic parenchyma (Fig. 1) and gallbladder (Fig. 2) in seven patients (7/7, 100%) in group 1, in the abdominal cavity in 10 patients (10/10, 100%) in group 2, and in the bile duct in three patients (3/7, 42.9%) in group 1 and four patients (4/10, 40%) in group 2. The location of each intrahepatic hematoma was related to an intrahepatic hypodense area, which implied parenchymal injury because of transhepatic interventional procedures.
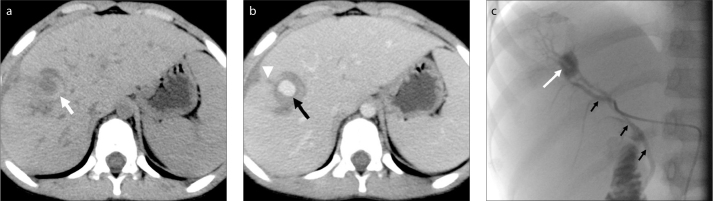
Figure 1.
a–c. A 13-year-old boy with hemobilia after percutaneous transhepatic biliary drainage (PTBD). Unenhanced abdominal CT image (a) shows a hematoma in the hepatic parenchyma (white arrow). Contrast-enhanced abdominal CT image (b) shows enhancement of a pseudoaneurysm (black arrow) near an injury to the hepatic parenchyma due to PTBD (white arrowhead). Selective angiogram of the right hepatic artery (c) shows a pseudoaneurysm in the segment V branch of the right hepatic artery (white arrow) and an arteriobiliary fistula (black arrows).
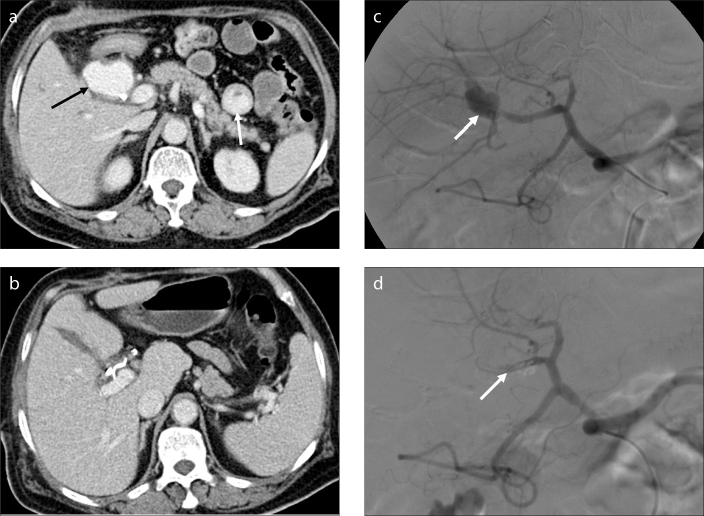
Figure 2.
a–c. A 58-year-old man with hemobilia after PTBD and biliary stent implantation. Unenhanced abdominal CT image (a) shows a hematoma (black arrow) in the gallbladder. Contrast-enhanced abdominal CT image (b) shows a small pseudoaneurysm (black arrow) near a hematoma within the parenchyma (white arrows), and hepatic parenchymal injury due to PTBD (white arrowheads). Superselective angiogram of the right hepatic artery (c) shows a small pseudoaneurysm in the subsegment VIII artery (white arrow) and an arteriobiliary fistula (black arrow).
The arterial phase of contrast-enhanced CT revealed a defined nodular lesion with an enhancement pattern that was suggestive of a pseudoaneurysm close to intrahepatic hematoma in six patients (6/7, 85.7%) in group 1 (Figs. 1b, 2b) and hepatic hilar in 10 patients (10/10, 100%) in group 2 (Fig. 3). Another diagnostic sign of hemobilia was extravasation of contrast material into the bile duct and small intestinal cavity in one patient (1/7, 14.3%) in group 1 and two patients (2/10, 20%) in group 2 (Fig. 3a). Other signs included dilation of the biliary system and enhancement of the bile ducts in three patients (3/7, 42.9%) in group 1 and six patients (6/10, 60%) in group 2, and arterial-phase hypoperfusion of the right lobe in three patients (3/10, 30%) with laparoscopic cholecystectomy.
Figure 3.
a–d. A 43-year-old man with hemobilia after laparoscopic cholecystectomy. Contrast-enhanced abdominal CT image (a) shows a large pseudoaneurysm (black arrow) and extravasation of contrast material into the small intestinal cavity (white arrow). Selective angiogram of the right hepatic artery (b) shows a pseudoaneurysm arising from the cystic artery ligation of the right hepatic artery (white arrow). Contrast-enhanced abdominal CT image after TAE (c) shows the disappearance of the pseudoaneurysm and coils in the hilar area. Selective angiogram of the right hepatic artery after embolization with gelatin sponge (1×1×1 mm) and coils (8–50 mm, 5–50 mm) (d) shows the absence of opacification of the pseudoaneurysm (white arrow).
The affected arteries included the intrahepatic arterial branches, right hepatic artery, and proper hepatic artery. In group 1 patients with transhepatic intervention, all affected arteries were intrahepatic segmental or subsegmental arteries; however, in group 2 patients who had undergone surgical procedures in the hilar area, all affected arteries involved the extrahepatic portion of the right hepatic artery or proper hepatic artery.
Table 1 shows the angiographic features of hemobilia, which included pseudoaneurysms in 22 cases (22/30, 73.3%) (Fig. 1c, 3c), simultaneous pseudoaneurysm and arteriobiliary fistula in three cases (3/30, 10%) (Fig. 2c), and extravasation of contrast material in five cases (5/30, 16.7%) (Fig. 4a). There was no significant difference in the angiographic features between the two groups (P = 0.127).
Table 1.
Angiographic features of 30 patients with hemobilia
| Group | PA | CE | PA+ABF | P |
|---|---|---|---|---|
| Transhepatic intervention (group 1) | 9 | 0 | 2 | 0.127 |
| Surgical procedures in hilar area (group 2) | 13 | 5 | 1 |
A two-tailed Fisher-Freeman-Halton test was used for analysis.
PA, pseudoaneurysm; CE, contrast extravasation; ABF, arteriobiliary fistula.
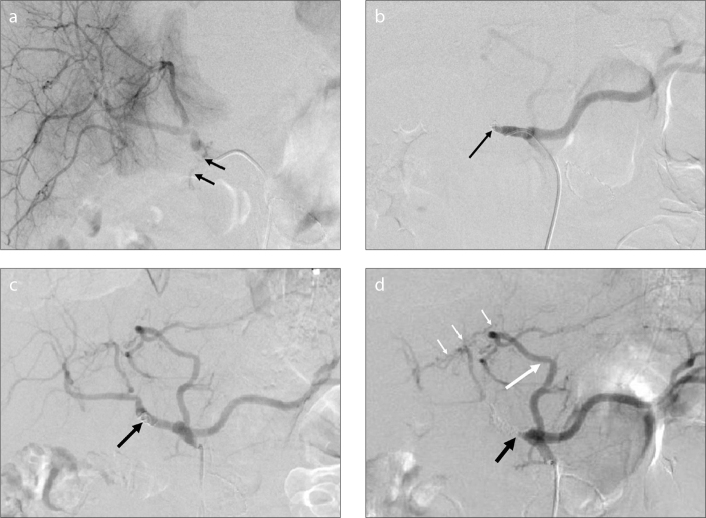
Figure 4.
a–d. A 58-year-old man with hemobilia after the resection of cholangiocarcinoma. Selective angiogram of the common hepatic artery (a) shows extravasation of contrast material through the common hepatic artery (black arrows). Selective angiogram after embolization with two coils (8–50 mm) (b) shows the absence of contrast extravasation and occlusion of the common hepatic artery (black arrow). Because of rebleeding three days after the first TAE, the second selective angiogram (c) shows recanalization of the common hepatic artery (black arrow). Selective angiogram of the celiac artery after embolization with coils (5–50 mm, 8–50 mm) (d) shows occlusion of the common hepatic artery (black arrow) and the accessory left hepatic artery (long white arrow) originating from the left gastric artery, which compensates for the blood supply to the hepatic parenchyma through the communicating vessels (short white arrows).
Eight patients from group 1 underwent distal embolization with an absorbable GS followed by proximal embolization of a segmental artery with coils (Table 2). Two patients underwent subsegmental embolization of intrahepatic arterial branches with PVA and one case was treated in areas proximal and distal to the injury site by coils. In group 2, an absorbable GS and coils were used for embolization of the right hepatic artery in one patient (Table 2; Fig. 3d), and the other 18 patients underwent proximal and distal arterial embolization of injured sites involving the right hepatic artery or proper hepatic artery using coils or microcoils (Table 2, Fig. 4b).
Table 2.
Embolic material used in 30 patients with hemobilia
| Group | PVA particles | GS+coils | Coils | P |
|---|---|---|---|---|
| Transhepatic intervention (group 1) | 2 | 8 | 1 | <0.001 |
| Surgical procedures in hilar area (group 2) | 0 | 1 | 18 |
A two-tailed Fisher-Freeman-Halton test was used for analysis.
PVA, polyvinyl alcohol; GS, gelatin sponge.
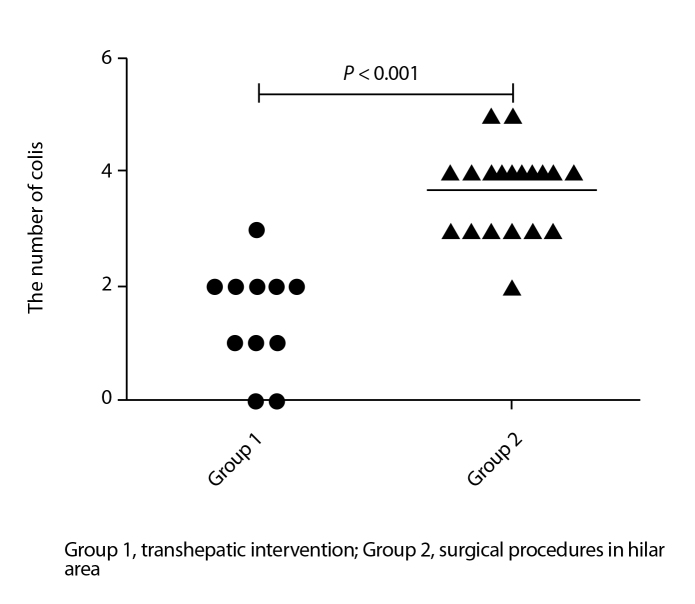
The embolic materials were significantly different between the two groups of patients (Table 2; P < 0.001). The mean number of coils also differed between the two groups, and the number of coils used in group 1 was less than that used in group 2 (Fig. 5; 1.45±0.93 vs. 3.68±0.75, P < 0.001).
Figure 5.
Scatter plot shows the total number of coils used in both groups. The number of coils in group 1 patients with transhepatic intervention is less than that used in group 2 patients with surgical procedures in the hilar area. The difference is significant between the two groups (P < 0.001).
Embolization was technically successful in all 30 patients (100%), and hemobilia stopped immediately after the first TAE. Three patients from group 2 experienced rebleeding on the third, seventh, and eighth days, respectively, after the first embolization using coils only, including one cholecystectomy case with embolization of the right hepatic artery and two cholangiocarcinoma cases with embolization of the proper hepatic artery. A second TAE was performed in three cases without subsequent rebleeding (Fig. 4c, 4d). The clinical success rate of the first embolization was 100% in group 1 vs. 84.2% in group 2 (P = 0.279); clinical success was also not significantly different between the embolic materials (P = 0.873).
Complications occurred in 63.6% of patients (7/11) in group 1 and 68.4% of patients (13/19) in group 2, and the complication rates were not significantly different between the two groups (P = 1.000) (Table 3). Neither ectopic embolism nor liver failure occurred in any patient. A hepatic abscess was found in one patient with status post-cholecystectomy. Eight patients (26.7%) were diagnosed with hepatic ischemia. Postembolization syndrome was found in 20 patients (66.7%). Four patients (13.3%) had a pleural effusion. Postoperative complications subsided with symptomatic treatment within 7–10 days. All patients were alive at follow-up and showed no sign of recurrence.
Table 3.
Complications related to TAE performed via intrahepatic (group 1) vs. extrahepatic (group 2) arteries
| Complication | Transhepatic intervention Group 1 n=11 |
Surgical procedures near hilum Group 2 n=19 |
P |
|---|---|---|---|
| Major | |||
| Class D hepatic abscess | 0 | 1* | |
| Class C pleural effusion | 2* | 2* | |
|
| |||
| Minor | |||
| Hepatic ischemia | 3* | 5* | |
| Postembolization syndrome | 7 | 13 | 1.000 |
Complications were categorized according to the SIR clinical practice guidelines.
A two-tailed Fisher’s exact test was used for analysis.
These patients also had postembolization syndrome, so complications occurred in 63.6% of patients (7/11) in group 1 and 68.4% of patients (13/19) in group 2.
Discussion
Two important diagnostic signs of hemobilia were found on the CT images in our study, including a hematoma within the gallbladder and/or bile duct and extravasation of contrast material into the bile duct and/or small intestine. Other signs included a hematoma within the hepatic parenchyma or abdominal cavity, enhancement of a pseudoaneurysm, dilation and enhancement of the bile duct, and hypoperfusion of the right lobe in the arterial phase.
In patients with suspected hemobilia, the initial investigation is upper gastrointestinal endoscopy, which may reveal blood flowing out of the papilla of Vater directly or indirectly, by the presence of fresh blood in the second part of the duodenum without an active site of bleeding (2, 3). However, 60% of hemobilia cases may be diagnosed by endoscopy (2), and further investigation may be required (4, 17). Contrast-enhanced CT images could provide diagnostic signs and be useful in determining the presence and location of a pseudoaneurysm before angiography. Although a pseudoaneurysm of the intrahepatic arteries is usually very small and not easily found, its location adjacent to an area of damaged liver parenchyma or the presence of an intrahepatic hematoma can aid in its identification. However, a pseudoaneurysm of the extrahepatic arteries is usually larger and easier to identify on CT images. Roudsari et al. (18) pointed to a declining role of angiography in the diagnosis of hemobilia and emphasized the importance of angiography more as a tool for management via embolization. Dilation and enhancement of the bile duct indicated inflammation of the bile duct due to repeated stimulation of the hemorrhage. Hepatic hypoperfusion in the arterial phase was due to compression of a large pseudoaneurysm of the right hepatic artery.
In this study, angiograms were able to locate the source of the bleeding in all cases. Subsegmental and segmental hepatic arteries were affected in patients with transhepatic intervention, and the right hepatic artery and proper hepatic artery were affected in patients who had undergone surgical procedures in the hilar area. These results also confirmed the iatrogenic causes of hemobilia. Previous studies have highlighted the failure of TAE in cases of hemobilia (2, 11). Angiography should be more selective, especially in cases of a possible anomalous hepatic artery, in addition to the usual celiac and superior mesenteric arteriography (9).
We observed five cases of hemobilia that showed only extravasation of contrast material on the angiogram, three cases that exhibited both a hepatic arteriobiliary fistula and a pseudoaneurysm, and 22 cases of pseudoaneurysm. The features on angiography did not differ between the two groups with different iatrogenic causes, which suggest that angiographic features are not associated with iatrogenic causes. A much higher incidence of pseudoaneurysm was noted in this study, which is in accordance with previous reports that showed that the development of a pseudoaneurysm around a damaged vessel commonly preceded the onset of hemobilia (2, 3, 7–10). Contrast extravasation and arteriobiliary fistula were uncommon and found in only a few cases, which might be related to the volume of contrast material used in this study and the injection pressure in angiography, or possibly to closure of the fistula between the bile duct and artery caused by clots from intermittent bleeding (3, 19).
Therapeutic options have evolved in recent years from the traditional surgical option toward a less invasive approach and include radiologic procedures such as ultrasound-guided compression, ultrasound-guided percutaneous thrombin injection, and endovascular management (embolization, stent-graft placement). The use of noninvasive treatment has led to a marked decrease in the morbidity and mortality rates for pseudoaneurysms (20). Endovascular treatment is considered as the first choice for the embolization of a visceral pseudoaneurysm (13, 20). In this study, TAE embolization was successfully performed with three types of embolic material, and the type of embolic material was chosen according to the size of the injured artery. The various embolic materials were highly effective in controlling bleeding during the early stage after embolization and our initial technical success rate using TAE was 100%. The main factors to be considered for successful embolization are the size of the vessels and duration of occlusion. In general, large vessels require coils; smaller vessels can be treated with PVA particles, a GS, cyanoacrylate, or microcoils (21). Cyanoacrylate is a permanent embolic agent and has been used in the treatment of selected traumatic injuries, especially in patients with coagulopathy (21). In this study, cyanoacrylate was not used for the TAE of hemobilia, because it was excessively costly and required extensive experience on the part of the operator to prevent serious complications (22).
Hepatic subsegmental embolization using PVA was successful in treating small pseudoaneurysms from subsegmental hepatic arterial branches in two of our patients. A problem with PVA particles is their tendency to aggregate and clump together, which can occur in a vessel that is more proximal than that intended, resulting in nontarget embolization, so it has seldom been used for embolization of injuries to the hepatic arteries (23, 24). However, our experience has indicated that PVA is effective in the embolization of hepatic subsegmental arterial injury, as long as there is sufficient attention to detail during the procedure.
Hepatic subsegmental or segmental embolization was performed in eight patients with intrahepatic arterial injury, initially using a GS, followed by coils. Embolization of the right hepatic artery was also successful in one post-cholecystectomy case using a GS initially, followed by coils. Previous research has shown that GS particles could be used for embolization of diffuse bleeding of hepatic arteries, but not for embolization of the middle branch alone, which could result in rebleeding after recanalization (25, 26). Owing to the small size of subsegmental or segmental hepatic arteries, distal embolization with a GS followed by proximal embolization with coils is an effective option (9). Our experience has also indicated that the combined application of GS particles and coils can successfully embolize an intrahepatic arterial injury, decrease the number of coils needed, and effectively control hemobilia in a more economic manner (21).
Besides the one patient with hemobilia with status post-cholecystectomy (group 2), who received GS particles and coils for embolization, 18 other patients with hemobilia (group 2) were embolized using coils. However, three of those patients experienced recurrent bleeding after initial embolization using coils only. This bleeding was controlled after additional long-range embolization with coils. We thought that the reason for all our failed embolizations was initial incomplete arterial occlusion. The arteries that exhibited rebleeding were larger branches with high flow, which should have undergone extensive, long-segment embolization of the artery proximal and distal to the point of bleeding; thus, repeat angiography showed recanalization of coils and recurrent bleeding. In addition, coils should have been sized 20%–30% larger than the size of the vessel as measured on a pre-deployment angiogram to prevent distal embolization or migration, as the placement of an undersized coil risks its distal embolization away from the intended location (24). Koganemaru et al. (27) reported that small-diameter primary coils and microcatheter tips that are thinner than normal can be used to increase the safety and reliability of coil embolization.
One patient developed a hepatic abscess after TAE; however, other serious procedure-related complications were not encountered in this study. Postembolization syndrome and a transient elevation in transaminase levels were noted in some patients. These symptoms disappeared after treatment. Previous studies have shown that hepatic segment and lobar embolization are relatively safe (6–9). Embolization of the common hepatic artery and proper hepatic artery should be performed carefully, particularly in patients with portal hypertension or severe liver function damage. Indirect portal vein angiography should be performed to ensure patency of the portal vein prior to TAE to avoid procedure-related liver necrosis and liver failure (2).
Our study had several limitations including its retrospective design and small sample size. In addition, only 17 patients underwent contrast-enhanced CT examinations prior to angiography. This study also compared the results of only two groups with different iatrogenic origins; we did not compare the results of treatment using different embolic materials in patients with the same iatrogenic origin of their injuries, which was due to the limited number of cases available.
In conclusion, CT can provide useful diagnostic information in hemobilia cases, and angiography remains an important method for effectively determining the vessel responsible for bleeding in hemobilia. Pseudoaneurysms, which are one of the most common angiographic features of hemobilia, were not associated with a specific iatrogenic origin but were seen in cases of both intrahepatic and extrahepatic injury. TAE proved to be an effective therapeutic method for the treatment of hemobilia, and successful embolization was performed with different embolic materials based on the iatrogenic origin and location of bleeding.
Main points.
CT can provide useful diagnostic information in cases with hemobilia.
Angiography is an important method for effectively assessing the responsible bleeding vessel in hemobilia.
Pseudoaneurysm, one of the most common angiographic features of hemobilia, was not associated with a specific iatrogenic origin but was seen in cases of both intrahepatic and extrahepatic injury.
Transcatheter arterial embolization, using different embolic materials based on the iatrogenic origin and bleeding location, is an effective therapeutic method for hemobilia.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
Financial disclosure
This study was supported by Scientific Research Project of Department of Education of Liaoning Province (L2012289) to FW.
References
- 1.Bloechle C, Izbicki JR, Rashed MY, et al. Hemobilia: presentation, diagnosis, and management. Am J Gastroenterol. 1994;89:1537–1540. [PubMed] [Google Scholar]
- 2.Murugesan SD, Sathyanesan J, Lakshmanan A, et al. Massive hemobilia: a diagnostic and therapeutic challenge. World J Surgery. 2014;38:1755–1762. doi: 10.1007/s00268-013-2435-5. http://dx.doi.org/10.1007/s00268-013-2435-5. [DOI] [PubMed] [Google Scholar]
- 3.Green MHA, Johnson CD, Jamieson NV. Haemobilia. Br J Surg. 2001;88:773–786. doi: 10.1046/j.1365-2168.2001.01756.x. http://dx.doi.org/10.1046/j.1365-2168.2001.01756.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida J, Donahue PE, Nyhus LM, et al. Hemobilia: review of recent experience with a worldwide problem. Am J Gastroenterol. 1987;82:448–453. [PubMed] [Google Scholar]
- 5.Cao H, Liu J, Li T, et al. Interventional therapy for the treatment of severe hemobilia after percutaneous transhepatic cholangial drainage: a case series. International Surg. 2013;98:223–228. doi: 10.9738/INTSURG-D-13-CC194. http://dx.doi.org/10.9738/INTSURG-D-13-CC194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatzidakis A, Petrakis J, Krokidis M, et al. Hepatic artery aneurysm presenting with hemobilia in a patient with Behçet’s disease: treatment with percutaneous transcatheteral embolization. Diagn Interv Radiol. 2006;12:53–55. [PubMed] [Google Scholar]
- 7.Rhim H, Lim HK, Kim YS, et al. Hemobilia after radiofrequency ablation of hepatocellular carcinoma. Abdom Imaging. 2007;32:719–724. doi: 10.1007/s00261-006-9158-0. http://dx.doi.org/10.1007/s00261-006-9158-0. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava DN, Sharma S, Pal S, et al. Transcatheter arterial embolization in the management of hemobilia. Abdom Imaging. 2006;31:439–448. doi: 10.1007/s00261-005-0392-7. http://dx.doi.org/10.1007/s00261-005-0392-7. [DOI] [PubMed] [Google Scholar]
- 9.Xu ZB, Zhou XY, Peng ZY, et al. Evaluation of selective hepatic angiography and embolization in patients with massive hemobilia. Hepatobiliary Pancreat Dis Int. 2005;4:254–258. [PubMed] [Google Scholar]
- 10.Teng WS, Wu RH, Chang JM, et al. Transcatheter arterial embolization for hemorrhage caused by injury of the hepatic artery. J Gastroenterol Hepatol. 2005;20:1062–1068. doi: 10.1111/j.1440-1746.2005.03768.x. http://dx.doi.org/10.1111/j.1440-1746.2005.03768.x. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson T, Travis S, Ettles D, et al. Hepatic artery angiography and embolization for hemobilia following laparoscopic cholecystectomy. Cardiovasc Intervent Radiol. 1999;22:20–24. doi: 10.1007/s002709900323. http://dx.doi.org/10.1007/s002709900323. [DOI] [PubMed] [Google Scholar]
- 12.Rivera-Sanfeliz GM, Assar OS, LaBerge JM, et al. Incidence of important hemobilia following transhepatic biliary drainage: left-sided versus right-sided approaches. Cardiovasc Intervent Radiol. 2004;27:137–139. doi: 10.1007/s00270-003-0022-0. http://dx.doi.org/10.1007/s00270-003-0022-0. [DOI] [PubMed] [Google Scholar]
- 13.Hemp JH, Sabri SS. Endovascular management of visceral arterial aneurysms. Tech Vasc Interv Radiol. 2015;18:14–23. doi: 10.1053/j.tvir.2014.12.003. http://dx.doi.org/10.1053/j.tvir.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Walter JF, Paaso BT, Cannon WB. Successful transcatheter embolic control of massive hematobilia secondary to liver biopsy. AJR Am J Roentgenol. 1976;127:847–849. doi: 10.2214/ajr.127.5.847. http://dx.doi.org/10.2214/ajr.127.5.847. [DOI] [PubMed] [Google Scholar]
- 15.Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 16.Choi SH, Gwon DI, Ko GY, et al. Hepatic arterial injuries in 3110 patients following percutaneous transhepatic biliary drainage. Radiology. 2011;261:969–975. doi: 10.1148/radiol.11110254. http://dx.doi.org/10.1148/radiol.11110254. [DOI] [PubMed] [Google Scholar]
- 17.Yokota J, Sugimoto T. Clinical significance of periportal tracking on computed tomographic scan in patients with blunt liver trauma. Am J Surg. 1994;168:247–250. doi: 10.1016/s0002-9610(05)80196-5. http://dx.doi.org/10.1016/S0002-9610(05)80196-5. [DOI] [PubMed] [Google Scholar]
- 18.Roudsari BS, Psoter KJ, Padia SA, et al. Utilization of angiography and embolization for abdominopelvic trauma: 14 years’ experience at a level I trauma center. AJR Am J Roentgenol. 2014;202:580–585. doi: 10.2214/AJR.13.11216. http://dx.doi.org/10.2214/AJR.13.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okazaki M, Ono H, Higashihara H, et al. Angiographic management of massive hemobilia due to iatrogenic trauma. Gastrointest Radiol. 1991;16:205–214. doi: 10.1007/BF01887347. http://dx.doi.org/10.1007/BF01887347. [DOI] [PubMed] [Google Scholar]
- 20.Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25(Suppl 1):173–189. doi: 10.1148/rg.25si055503. http://dx.doi.org/10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 21.Lopera JE. Embolization in trauma: principles and techniques. Semin Intervent Radiol. 2010;27:14–28. doi: 10.1055/s-0030-1247885. http://dx.doi.org/10.1055/s-0030-1247885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavili E, Donmez H, Ozcan N, Akcali Y. Endovascular treatment of lower limb penetrating arterial traumas. Cardiovasc Intervent Radiol. 2007;30:1124–1129. doi: 10.1007/s00270-007-9142-2. http://dx.doi.org/10.1007/s00270-007-9142-2. [DOI] [PubMed] [Google Scholar]
- 23.Chimpiri AR, Natarajan B. Visceral arteriography in trauma. Semin Intervent Radiol. 2009;26:207–214. doi: 10.1055/s-0029-1225669. http://dx.doi.org/10.1055/s-0029-1225669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol. 2008;25:204–215. doi: 10.1055/s-0028-1085930. http://dx.doi.org/10.1055/s-0028-1085930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Z, Yan S, Zhou X, et al. Hepatic artery angiography and embolization for hemobilia after hepatobiliary surgery. Chin Med J (Engl) 2001;114:803–806. [PubMed] [Google Scholar]
- 26.Eurvilaichit C. Iatrogenic hemobilia: management with transarterial embolization using Gelfoam particles. J Med Assoc Thai. 1999;82:931–937. [PubMed] [Google Scholar]
- 27.Koganemaru M, Abe T, Nonoshita M, et al. Management of visceral artery embolization using 0.010-inch detachable microcoils. Diagn Interv Radiol. 2014;20:345–348. doi: 10.5152/dir.2014.13382. http://dx.doi.org/10.5152/dir.2014.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]