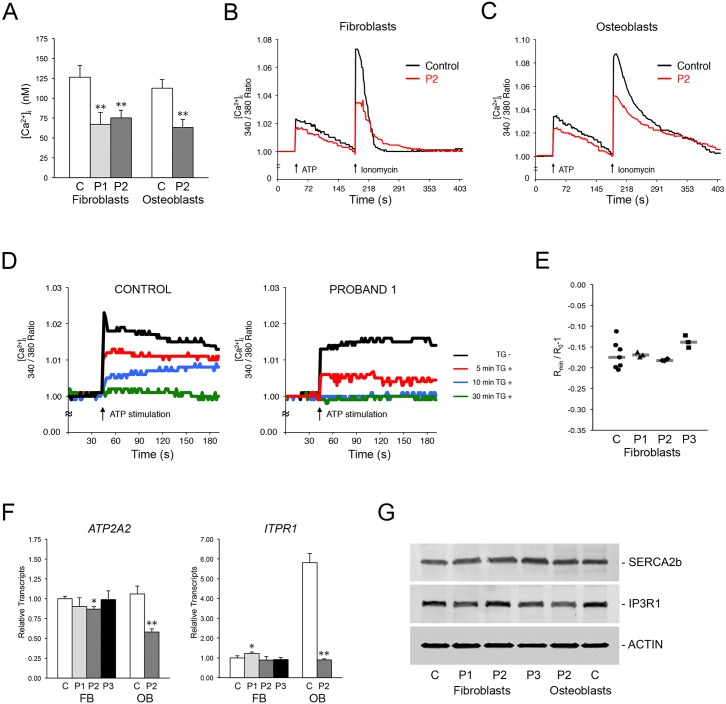
Fig 3. Absence of TRIC-B dysregulates ER Ca2+ homeostasis.
(A) Basal levels of [Ca2+]i in control (C) and proband fibroblasts (P1, P2) and osteoblasts (P2). (B) Decreased Ca2+ mobilization in TRIC-B deficient fibroblasts (red lines). ATP- and Ionomycin-stimulated Ca2+ release, as well as the return to baseline levels, are decreased in proband (P2) cells. (C) Decreased Ca2+ mobilization in TRIC-B deficient osteoblasts (red lines). (D) The intracellular Ca2+ stores available for IP3R-mediated release are more rapidly depleted in Proband 1 fibroblasts (right) versus normal control cells (left). ATP-stimulated Ca2+ release is abrogated within 10 minutes in proband cells compared to 30 minutes in control cells following inhibition of SERCA channels with thapsigargin (TG). (E) Measurement of ER luminal Ca2+ using ER-localized Ca2+ indicator. Each point represents the average of one measurement containing 1–7 cells. The difference between the steady-state and Ca2+-depleted FRET signal emitted by the D1ER chameleon class Ca2+ sensor was equivalent in normal control and proband cells. (F) Quantitative RT-PCR in control (C) and proband (P1, P2, P3) cells. There is no significant difference in expression levels of SERCA2 (ATP2A2) and IP3R1 (ITPR1) in fibroblasts, but transcripts are significantly reduced in proband (P2) osteoblasts. (G) Immunoblots of control (C) and proband (P1, P2, P3) cell lysates demonstrate equivalent levels of SERCA2b and IP3R1 Ca2+ channels. *, p < 0.05; **, p < 0.01.