Fig. 3. Comparison of custom tapered microwells fabricated using diffuser back-side lithography for CTC cluster assay and conventional cylindrical microwells.
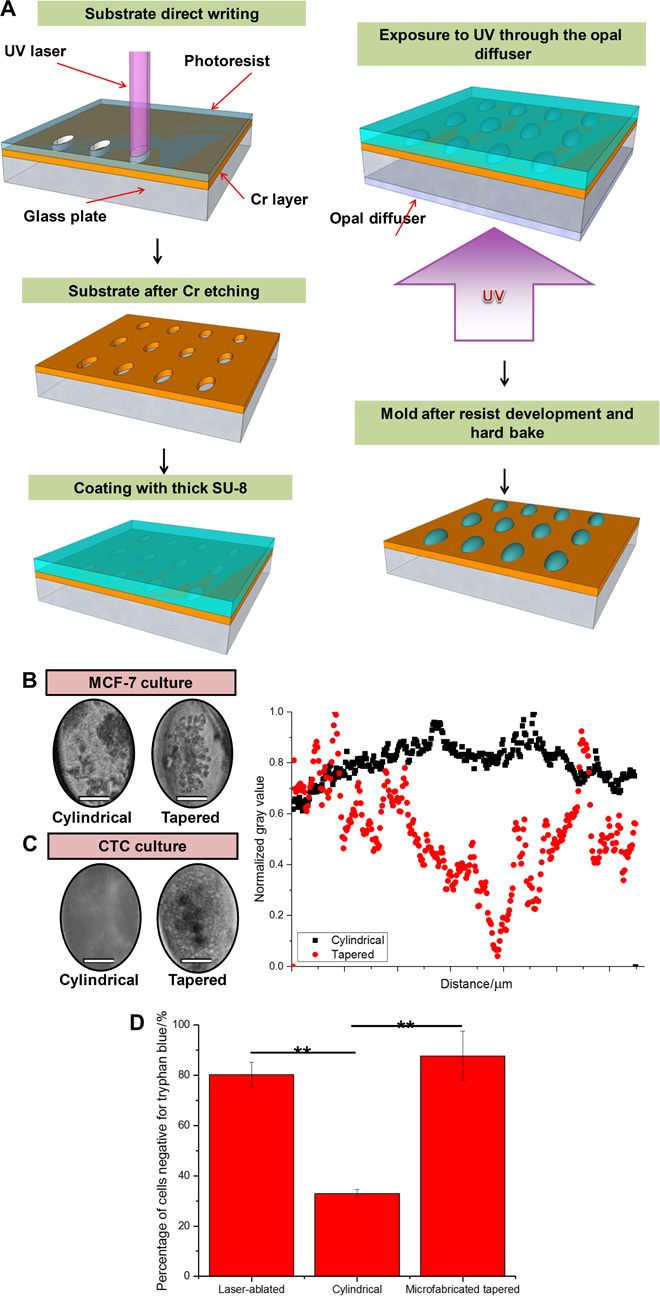
(A) Fabrication procedure: elliptical openings defining the size and position of the wells created on a soda-lime optical mask blank by laser direct writing. Subsequent Cr etching and stripping of the remaining resist were followed by coating with a layer of SU-8 2100 resist. Ultraviolet (UV) exposure to obtain pillar-like structures with the elliptical footprint and elliptical cross-shaped structures. Postbaking, ultrasound bath, and hard baking resulted in a template ready for PDMS molding. (B) (Left) Culture of MCF-7 in custom tapered microwells and cylindrical microwells. Single clusters were consistently established with tapered microwells. Scale bars, 50 μm. (C) (Left) Culture of clinical blood samples in custom tapered microwells and cylindrical microwells. Only debris was formed in cylindrical microwells. Scale bars, 50 μm. (Right) Combined scatterplots of gray values, with values normalized to the highest count for a particular microwell. Cylindrical microwells did not generate clusters, whereas tapered microwells led to a single dense cell cluster as observed from the region of low gray value. (D) Bar graph presenting results from a viability assay using trypan blue staining. Percentage of cells negative for trypan blue (viable cells) was significantly lower in the sample portion cultured in cylindrical microwells. All error bars represent SD of triplicate cultures from different samples. **P < 0.01.
