Abstract
Background
Dry mouth is known to cause severe pain in the intraoral structures, and many dry mouth patients have been suffering from intraoral pain. In development of an appropriate treatment, it is crucial to study the mechanisms underlying intraoral pain associated with dry mouth, yet the detailed mechanisms are not fully understood. To evaluate the mechanisms underlying pain related to dry mouth, the dry-tongue rat model was developed. Hence, the mechanical or heat nocifensive reflex, the phosphorylated extracellular signal-regulated kinase and phosphorylated GluR1-IR immunohistochemistries, and the single neuronal activity were examined in the trigeminal spinal subnucleus caudalis of dry-tongue rats.
Results
The head-withdrawal reflex threshold to mechanical, but not heat, stimulation of the tongue was significantly decreased on day 7 after tongue drying. The mechanical, but not heat, responses of trigeminal spinal subnucleus caudalis nociceptive neurons were significantly enhanced in dry-tongue rats compared to sham rats on day 7. The number of phosphorylated extracellular signal-regulated kinase-immunoreactive cells was also significantly increased in the trigeminal spinal subnucleus caudalis following noxious stimulation of the tongue in dry-tongue rats compared to sham rats on day 7. The decrement of the mechanical head-withdrawal reflex threshold (HWT) was reversed during intracisternal administration of the mitogen-activated protein kinase kinase 1 inhibitor, PD98059. The trigeminal spinal subnucleus caudalis neuronal activities and the number of phosphorylated extracellular signal-regulated kinase-immunoreactive cells following noxious mechanical stimulation of dried tongue were also significantly decreased following intracisternal administration of PD98059 compared to vehicle-administrated rats. Increased number of the phosphorylated GluR1-IR cells was observed in the trigeminal spinal subnucleus caudalis of dry-tongue rats, and the number of phosphorylated GluR1-IR cells was significantly reduced in PD98059-administrated rats compared to the vehicle-administrated tongue-dry rats.
Conclusions
These findings suggest that the pERK-pGluR1 cascade is involved in central sensitization of trigeminal spinal subnucleus caudalis nociceptive neurons, thus resulting in tongue mechanical hyperalgesia associated with tongue drying.
Keywords: Tongue pain, dry mouth, medulla, phosphorylated extracellular signal-regulated kinase, phosphorylated GluR1, caudalis
Background
Dry mouth is one of the oral symptoms frequently associated with various oral diseases such as Sjögren’s syndrome, salivary gland calyces, inflammation, and tumors.1,2 A variety of sensory or motor dysfunction can be induced in oral tissues of dry mouth patients.3 These include tongue pain, which is one of the most severe symptoms in dry mouth patients, disturbing food intake, mastication, and oral sensory functions.4,5 Thus, to develop an appropriate treatment, it is essential to elucidate the mechanisms underlying tongue pain associated with tongue drying.
It is well known that trigeminal spinal subnucleus caudalis (Vc) is the key nucleus, that relays orofacial noxious sensory information to the higher central nervous system.6 Alteration of molecular expression in Vc neurons, similar to trigeminal ganglion (TG) neurons, occurs in animals with orofacial inflammation or trigeminal nerve injury.7–9 In fact, the extracellular signal-regulated kinase (ERK) phosphorylation occurs in Vc neurons within 5 min following various orofacial noxious stimuli, and the number of phosphorylated ERK-immunoreactive (pERK-IR) cells is increased following graded mechanical or heat stimulation.9–11 It has also been reported that ERK phosphorylation is involved in hyperexcitability of nociceptive neurons in the spinal dorsal horn (SDH) following repeated high intensity-electrical stimulation of the sciatic nerve.12 These findings indicate that the ERK phosphorylation in Vc neurons is in fact involved in the enhancement of nociceptive neuronal excitability.
The Arufa-amino-3-hydrozy-5methylisoxazole-4-propionic acid receptor (AMPAR) is composed of four subunits (glutamate receptor subunits [GluR] 1-4) and has an important role in excitatory synaptic transmission.13–15 These subunits form tetramers that are functionally identical to the synaptic transmission. The GluRs can be phosphorylated by various stimuli. The phosphorylated GluRs are involved in a variety of functions such as localization of channels, conductance of membrane, and probability of channel opening.15 For instance, the phosphorylated GluR1 (pGluR1) subunit is involved in AMPAR membrane trafficking and fast synaptic transmission, which is associated with glutamate binding.16,17 It has also been reported that GluR1 phosphorylation is involved in the enhancement of excitatory synaptic transmission in the early phase of long-term potentiation in the SDH neurons in neuropathic rats.18
These data strongly suggest that the AMPAR, including its pGluR1 subunit, contributes to the sensitization of Vc nociceptive neurons and thereby to the tongue pain associated with tongue drying. Therefore, we hypothesized that the functional interaction between pERK and pGluR1 in Vc nociceptive neurons may be involved in tongue pain associated with tongue drying. To investigate the mechanisms underlying tongue pain associated with tongue drying in rats, we examined the mechanical or heat nocifensive reflex, the pERK and pGluR1 immunohistochemistries (IHCs) and the single neuronal activity in the Vc in dry-tongue rats.
Methods
Animals
This study was approved by the Animal Experimentation Committee at Nihon University, and experimental procedures were performed according to the guidelines of the International Association for the Study of Pain.19 Adult male Sprague-Dawley rats weighing 200–260 g (Japan SLC, Shizuoka, Japan) were used and were maintained in a temperature-controlled room (23℃) with a 12/12 h light-dark cycle. The rats were raised under pathogen-free conditions and fed ad libitum. All efforts were made to minimize animal suffering and reduce the number of animals used.
Dry-tongue rats
Rats were lightly anesthetized with 2% isoflurane (Mylan, Canonsburg, PA, USA) with dry ambient air in the plastic chamber. Tongue was gently pulled out from the mouth and kept in the dry ambient air for 2 h every day for 14 days (dry-tongue rats). Sham rats were similarly anesthetized for 2 h every day for 14 days, but without tongue drying in the ambient.
Nocifensive reflex threshold measurement
The HWT was measured under anesthesia with 2% of isoflurane (Mylan) in oxygen.11,20 Head-withdrawal reflex was visually identified following mechanical or heat stimulation of the tongue. The threshold intensities for evoking HWT to mechanical or heat stimulation of the lateral edge of tongue (3 mm posterior from tip of tongue) were measured in dry-tongue and sham rats under the light anesthesia with 2% isoflurane. We checked the depth of the anesthesia by applying quantified mechanical stimulus to the hind paw, which causes weak paw withdrawal reflex, before application of the mechanical or heat stimuli to the tongue, and paw withdrawal reflex threshold (PWT) was defined. The PWT was used as the indicator of the depth of anesthesia, and PWT was kept same value as that before application of mechanical stimuli to the tongue by adjustment of isoflurane concentration during HWT measurement.
Mechanical stimulation (0–150 g, 10 g/s, cut off: 150 g) was applied to the lateral edge of the left side tongue by using forceps with flat tips (4 mm2; Panlab, S.L., Barcelona, Spain) in lightly anesthetized rats. The stimulus velocity was manually controlled consecutively (10 g/s) according to the guided track line appeared on the microcomputer screen, and the mechanical intensity eliciting head withdrawal was defined as the mechanical HWT.
Heat stimulation (35℃–60℃, 1℃/s, cut off: 60℃) was also applied to left side of the tongue using a contact heat probe (9 mm2; Intercross, Tokyo, Japan) under the light anesthesia. The threshold temperature for eliciting head withdrawal was defined as the heat HWT.21 The mechanical or heat stimulation was applied three times with 5 min intervals, and the mean value of the HWTs were determined.
The HWTs were measured 2 h after tongue drying or sham treatment. The baseline HWTs to mechanical and heat stimulation were measured before tongue drying or sham treatment. The HWTs were measured on days 3, 7, 10, and 14 during tongue drying or sham treatment (dry/mechanical: n = 7, sham/mechanical: n = 7, dry/heat: n = 5, sham/heat: n = 5). In inhibitor administration experiments, the mitogen-activated protein kinase kinase 1 (MEK1) inhibitor PD98059 or vehicle was administrated from days 1 to 7, and the HWT to mechanical stimulation was also measured in same time course (dry vehicle: n = 6, dry PD98059: n = 5). The baseline HWT value was indicated as 100%.
Intracisternal administration of PD98059
The procedure for cisternal catheter insertion was similar to our previous study.8 Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and were placed in a stereotaxic frame. The occipital bone was exposed, and a small hole was drilled to insert a polyethylene tube (0.8 mm in diameter, Natsume, Tokyo, Japan) approximately 5 mm into the cisterna magna. The tube was attached to the occipital bone with instantaneous adhesive immediately and was fixed to skull with stainless steel screw and dental resin. The tube was connected with osmotic mini pump (Alzet model 2001, Durect Corporation, Cupertino, CA, USA) and was placed under the skin of the back of the rats and incised skin was sutured. The total drug infusion volume of the pump was 168 µl and the infusion rate was 1 µl/h for 7 days. The rats were allowed to recover for one day before experiments were performed. PD98059 was initially dissolved in 20% dimethyl sulfoxide (DMSO) at a concentration of 1 µg/μl (3.7 mM) as stock solution, and then further diluted to 0.1 µg/μl in 10% DMSO for i.c. injection and 10% DMSO was used as the vehicle.22
Experimental design for IHC
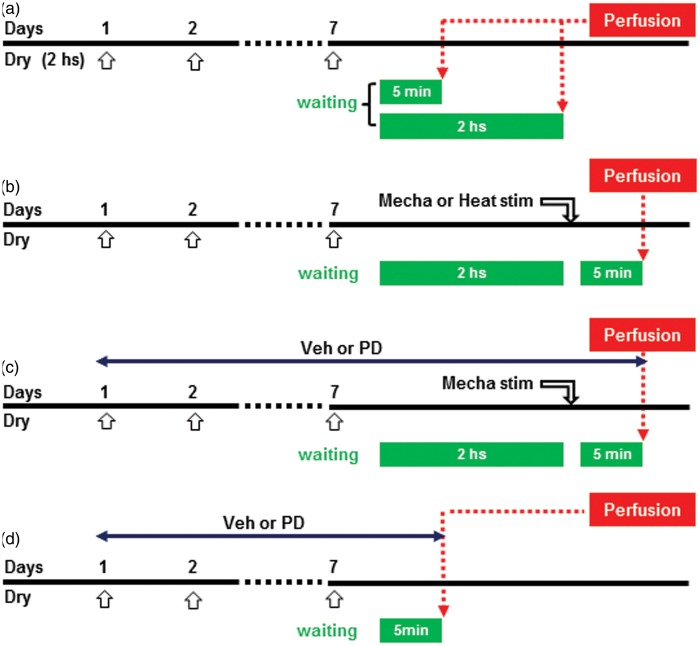
IHC experiments were conducted in dry tongue and sham rats receiving mechanical or heat stimulation on day 7. Four groups of rats were introduced for IHCs as follows: (1) The rats were perfused at 5 min (dry: n = 5, sham: n = 5) or 2 h (dry: n = 5, sham: n = 5) after dry tongue or sham treatment on day 7, and then sections were processed for pERK IHC (Figure 1(a)). (2) The rats were perfused at 5 min after noxious mechanical (dry: n = 5, sham: n = 5) or heat (dry: n = 5, sham: n = 5) stimulation at 2 h after dry tongue or sham treatment on day 7, and sections were processed for pERK IHC. The noxious mechanical stimulation to the tongue (intensity: 150 g pinch, duration: 20 s, interval: 10 s, number of applications: 10 times) was applied using forceps. Heat stimulation to the tongue (intensity: 50℃, duration: 60 s, interval: 10 s, number of stimulus: five times) was applied using heat probe (Figure 1(b)). (3) The rats were perfused at 5 min after noxious mechanical stimulation (dry vehicle: n = 5, dry PD98059: n = 5) at 2 h after dry tongue or sham treatment on day 7 with continuous i.c. PD98059 or vehicle administration, and sections were processed for pERK (Figure 1(c)). (4) This experiment assessed pGluR1 expression and the effect of PD98059 or vehicle i.c. administration on GluR1 phosphorylation in dry-tongue rats on day 7 (sham: n = 6, dry vehicle: n = 7, dry PD98059: n = 7). The rats were perfused at 5 min after dry tongue or sham treatment on day 7 (Figure 1(d)).
Figure 1.
Experimental designs for immunohistochemistry. (a) Design for testing the effect of tongue dryness on ERK phosphorylation in dry-tongue rats. (b) The effect of mechanical or heat stimulation in tongue dryness on ERK phosphorylation in dry-tongue rats. (c) The effect of PD administration on ERK phosphorylation by mechanical stimulation in dry-tongue rats. (d) The effect of PD administration on GluR1 phosphorylation in dry-tongue rats. Open arrows: daily tongue drying or sham treatment for 2 h. Veh: vehicle, PD: PD98059 in this and following figures.
pERK and pGluR1 IHCs
Rats were transcardially perfused with saline followed by a fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) under sodium pentobarbital (50 mg/kg, i.p.) anesthesia. The lower brainstem and upper cervical spinal cord were removed and post-fixed with same fixative for 48 h at 4℃, and then kept in 0.01 M phosphate-buffered saline (PBS) containing 20% sucrose (w/v) for 24 h at 4℃ for cryoprotection. Transverse sections (30 µm) were cut on a freezing microtome and every fourth section was corrected in 0.01 M PBS. For pERK IHC, free-floating tissue sections were incubated in 3% normal goat serum (NGS) in 0.3% triton X in 0.01 M PBS for 2 h at room temperature (RT), and then incubated in rabbit anti-phospho-p44/42 MAPK (Thr202/Tyr204) antibody (1:1000; Cell Signaling, Beverly, MA, USA) for 72 h at 4℃. The sections were then incubated in biotinylated goat anti-rabbit IgG (1:600; Vector Laboratories, Burlingame, CA, USA) for 2 h at RT. After rinsing, the sections were incubated in peroxidase-conjugated avidin-biotin complex (1:100; Vector Laboratories) for 1 h at RT. They were then washed in 0.05 M Tris buffer (TB), and next incubated in 0.035% 3.3′-diaminobenzidine-tetra HCl, 0.2% nickel ammonium sulfate, and 0.05% peroxide in 0.05 M TB (pH 7.4). The sections were then washed in 0.01 M PBS and mounted on MAS-GP coated slides (Matsunami, Tokyo, Japan), air-dried and dehydrated in a series of alcohols (from 50% to 100%) and then cover slipped with mounting medium (Eukitt, O. Kindler, Germany). Under bright-field illumination, pERK-IR cells appeared as homogenous, gray-black elements with a well-defined border. The number of pERK-IR cells was counted from every fourth sections, and the mean number of pERK-IR cells from six sections in each level of brain stem was calculated in each rat. Specific staining was abolished by omission of primary antibody.
Sections were incubated in 2% bovine serum albumin in 0.3% triton X in 0.01 M PBS for 12 h at 4℃ for pGluR1 IHC, in 3% NGS in 0.3% triton X in 0.01 M PBS for pERK, grail fibrillary acidic protein (GFAP) and neuronal nuclei (NeuN) IHCs for 2 h at RT, or in 3% normal donkey serum in 0.3% triton X in 0.01 M PBS for ionized calcium-binding adaptor molecule-1 (Iba1) IHC for 2 h at RT (n = 1 in each), and then incubated in anti-rabbit phospho-GluR1 antibody (1:200; Cell signaling Technology) for four days at 4℃. For double IHCs, they were incubated in rabbit anti-phospho-p44/42 MAPK (Thr202/Tyr204) antibody (1:300) for three days at 4℃ and mouse anti-NeuN antibody (1:1000; Millipore, Billerica, MA) for 24 h at 4℃, mouse anti-GFAP (1:500; Millipore), or goat anti-Iba1 (1:500; Abcam, Cambridge) for three days at 4℃. For pGulR1, IHC sections were then incubated in secondary antibodies anti-rabbit Alexa Fluor 568 IgG (1:200; Invitrogene, Eugene, OR) for 2 h at RT in the dark room. For double IHCs, sections were incubated in goat Alexa Fluor 568 IgG (1:200; Invitrogene, Eugene, OR) or donkey anti-rabbit Alexa Fluor 488 IgG (1:200; Invitrogene, Eugene, OR), goat anti-mouse Alexa Fluor 488 IgG (1:200; Invitrogene, Eugene, OR), and donkey anti-goat Alexa Fluor 568 IgG (1:200; Invitrogene, Eugene, OR) for 2 h at RT in the dark room. After that, sections were washed in PBS three times for 10 min and mounted on slides and cover slipped with PermaFluor (Thermo scientific, Fremont, CA). The photomicrographs of pGluR1-IR and double IHCs cells were taken using confocal microscope (Carl Zeiss Japan Inc., Tokyo, Japan). The number of pGluR1-IR cells was counted from every fourth sections in the square areas of the dorsal portion of Vc (588 × 222 µm2) in which pGluR1-IR cells were observed under fluorescent microscope (BZ9000, Keyense, Osaka, Japan), and the mean number of pGluR1-IR cells from six sections in each level of brain stem was calculated in each rat.
Neuron recording
Neuron-recording experiments were carried out dry tongue or sham-treated rats on day 7 (sham: n = 9, dry vehicle: n = 11, dry PD98059: n = 10). Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). Catheters were positioned in the right femoral vein for pancuronium bromide infusion (1 mg/kg/h) during recording session. After tracheotomy, rats were respired artificially with oxygen-enriched room air and isoflurane (1.0%–2.0%). The expired CO2 concentration was monitored and maintained between 3.0% and 4.0%. Rectal temperature was maintained at 37.0℃–38.0℃ by a thermostatically controlled heating pad (FST INC., Foster City, CA, USA), and the electrocardiogram was monitored. When the heart beat rate increased more than 300/min, the isoflurane concentration increased appropriately.
The rats were mounted in a stereotaxic frame, the medulla was exposed, and a mineral oil pool was made with skin flaps surrounding the laminectomy. Single neuron activity was recorded from the dorsal portion of Vc approximately obex level which pERK-IR cells were observed (see Figure 7(a)) by directing the electrode at an angle of 10° off vertical. The recording site was confirmed from the location of the electrolytic lesion (10 μA, 10 s) after recording. Extracellular unit activity was recorded using tungsten microelectrodes (impedance: 10–12 MΩ, FHC, ME, USA).
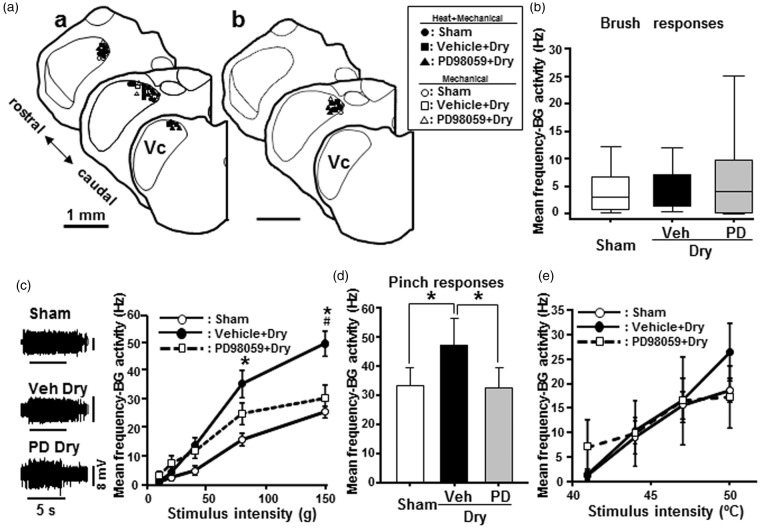
Figure 7.
Vc neuron activities on day 7 after drying of the tongue. Single neuron activities of nociceptive neurons in the Vc. (a) Recording sites of nociceptive neurons. (a)a: WDR neurons. (a)b: NS neurons. (b) Mean firing frequency to brush stimuli (sham: n = 15, tongue drying: n = 18, PD-administrated tongue dry: n = 10). (c) Typical responses of Vc WDR neurons to noxious mechanical stimulation (150 g) of the RFs in sham (n = 15), vehicle-administrated dry-tongue (n = 18), and PD98059-administrated dry-tongue rats (n = 10). Mean firing frequency to graded pressure stimuli. (d) Mean firing frequency to pinch stimuli (sham: n = 15, tongue drying: n = 17, PD-administrated tongue dry: n = 10). (e) Mean firing frequency to graded heat stimuli (sham: n = 9, tongue drying: n = 11, PD-administrated tongue dry: n = 13). PD98059 + Dry: PD98059-administrated dry-tongue rats. −BG activity: minus background activity. *p < 0.05 (vs. 10 g stim), #p < 0.05 (vs. sham or PD98059 + Dry) in panel (c). *p < 0.05 in panel (d).
Vc neurons were searched by applying mechanical stimulation (brush or pressure) to the tongue. When a single neuron was isolated, mechanical stimulation was applied to the tongue mucosa and tongue receptive field (RF) was mapped. Mechanical stimuli consisted of brush with a camel hair brush, graded pressure produced by blunt forceps, and pinch with small arterial clip. In order to avoid sensitization due to repeated stimuli, noxious mechanical stimuli were applied to only small area of the RF in each neuron. Nociceptive neurons were classified as wide dynamic range (WDR) and nociceptive specific (NS) neurons according to mechanical responses; WDR neurons respond to non-noxious and noxious stimuli and NS neurons respond to noxious stimuli. When a mechanical responsive neuron was identified, brush by camel blush, pressure by digital blunt forceps, pinch by small arterial clip and heat (Intercross, Tokyo, Japan) stimuli were applied to the left tongue. Brush stimulation was applied on the surface of the tongue five times (1 Hz) and graded pressure stimulation was applied by digital blunt forceps with 1 min intervals (10, 20, 40, 80, and 150 g). The increment velocity of stimulus intensities was manually controlled at a speed of 10 g/s. Pinch stimulation was applied to the center of the RFs for 5 s. Heat stimulation was applied by a contact heat probe (5 mm in diameter). The increment rate of temperature was set at 3℃/s. Before application of the thermal stimulus to the tongue, the surface temperature was adapted to 35℃ for 180 s. Heat stimuli were applied to the center of the RFs with 120 s interstimulus intervals (41, 44, 47, and 50℃).
Neuronal responses were fed into a hard disc for subsequent analysis of signals. The waveform of single neuronal activities was analyzed offline. The waveform of each neuron was identified using Spike 2 software (CED, Cambridge, UK). Peristimulus time histograms (PSTH, bin width = 1 s) were generated in response to each stimulus. Background activity (BG) was first recorded for 10 s before application of mechanical or thermal stimulus and then subtracted from the evoked responses during the subsequent analysis. The mean firing frequency was calculated during mechanical or thermal stimulation, and stimulus-response functions of each Vc neuron were obtained in response to the mechanical or thermal stimuli to the tongue. The mechanical or thermal stimulation of the RF was considered to have induced an effect when the peak firing frequency during 5 s mechanical or 30 s (with 60 s interstimulus intervals) heat stimulation differed from the mean background discharge rate by ±2 SD (standard deviation).
At the end of each experiment, the rat was overdosed with sodium pentobarbital (80 mg/kg, i.p.) and sacrificed. The brain was removed and placed in cold fixative (4% PFA in 0.01 M PBS) for a few days, then transferred to cold phosphate-buffered 20% sucrose for 48 h. Serial sections (50 µm) were cut along the path of the electrode penetration. The sections were counter-stained with thionine for identification of recording sites. Recording sites were drawn using Camera lucida drawing tube (Neurolucida 2000, MicroBrightField, Williston, VT).
Statistical analysis
The present data are shown as mean ± SEM. The two-way analysis of variance (ANOVA) followed by Bonferroni test was performed on the HWTs at each time point during the treatment. For comparison of mechanical nocifensive behavior between vehicle-administrated and PD98059-administrated dry-tongue rats, two-way repeated measures ANOVA followed by Tukey test was used. The one way ANOVA followed by Tukey test was also used for comparison of HWT measurement between pre- and post-tongue drying. Student t test was used to compare the number of pERK-IR cells between dry tongue and sham rats, to compare the number of pERK-IR cells between vehicle-administrated and PD98059-administrated dry-tongue rats. The one-way ANOVA followed by Tukey test was performed to compare the number of pGluR1-IR cells between vehicle-administrated dry tongue, PD98059-administrated dry tongue and sham rats. Two Way ANOVA on Rank was used to compare firing frequency following brush stimulation. Two way repeated measures ANOVA following Tukey test was used to compare the firing frequency following graded mechanical and heat stimuli between vehicle-administrated dry tongue, PD98059-administrated dry tongue and sham rats. Differences were considered significant at p < 0.05. One way ANOVA following Holm-Sidak method was used to compare the frequency following pinch stimulation between vehicle-administrated dry tongue, PD98059-administrated dry tongue and sham rats.
Results
HWT to mechanical and heat stimuli
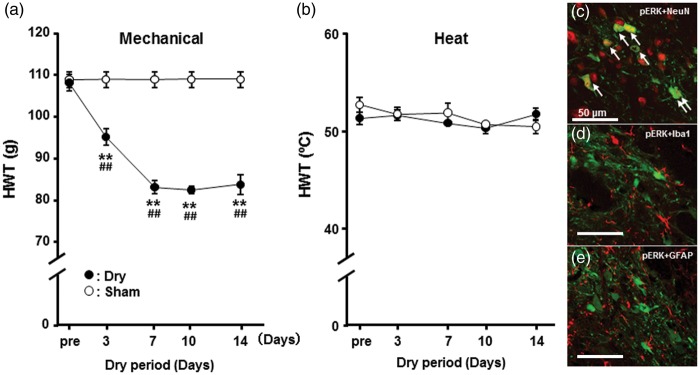
The HWT to mechanical stimulation of the tongue was significantly decreased compared to pretreatment value in dry-tongue rats and was significantly reduced in dry-tongue rats compared to sham rats on days 3, 7, 10, and 14 (Figure 2(a)). On the other hand, we did not observe any changes in HWT values to heat stimulation of the tongue in dry-tongue rats throughout the experimental period (Figure 2(b)). Furthermore, sham rats showed no change in HWT values to mechanical or heat stimulation of tongue during the observation period.
Figure 2.
Time-course change in mechanical or heat HWT in dry-tongue or sham rats. (a) Mechanical HWT. (b) Heat HWT. Mechanical but not heat HWT was significantly reduced on days 3–14 in dry-tongue rats. (c), (d), and (e) Merged photomicrographs of pERK-IR cells (green) and NeuN-IR cells (red) (c), pERK-IR cells (green), and GFAP-IR cells (red) (d) and pERK-IR cells (green) and Iba1-IR cells (red) (e). Arrows indicate pERK-IR cells merged with NeuN-IR cells. Dry: dry-tongue rats in this and following figures. **p < 0.01 (vs. pre), ##p < 0.01 (vs. sham).
ERK phosphorylation in Vc neurons
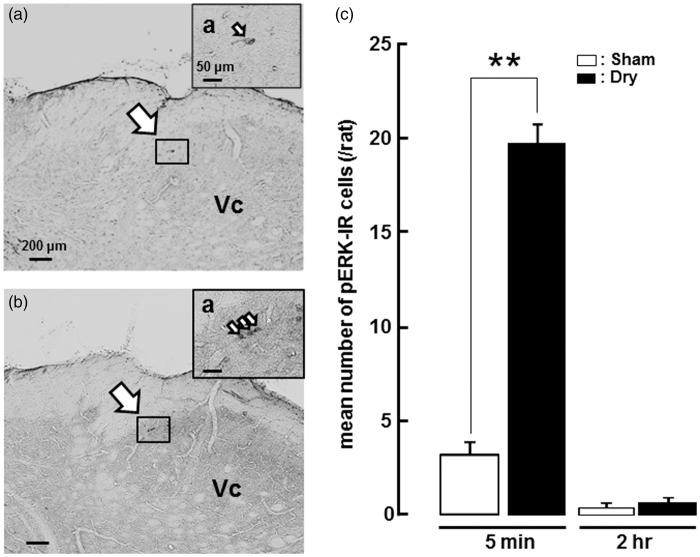
We studied phosphorylation of extracellular signal-regulated kinase (pERK) in Vc neurons in dry-tongue and sham rats with noxious mechanical (150 g) or heat (50℃) stimulation of tongue on day 7. The pERK-IR cells we observed also showed NeuN-IR but not GFAP- and Iba1-IR, indicating that pERK-IR cells in this study were defined as neurons in (Figure 1(c)–(e)). A small number of pERK-IR cells was observed in the superficial laminae of the dorsal portion of the Vc, in sham rats 5 min after cessation of sham treatment on day 7 (Figure 3(a)). However, many pERK-IR cells were observed in the superficial laminae of the Vc in dry-tongue rats 5 min after cessation of the tongue drying on day 7 (Figure 3(b)). The number of pERK-IR cells was significantly larger in the dry-tongue rats compared to sham rats perfused 5 min after tongue-dry treatment (Figure 3(c)). Furthermore, the number of pERK-IR cells was not significantly different between sham and dry-tongue rats perfused 2 h after tongue drying on day 7. These findings indicate that tongue drying causes ERK phosphorylation in the Vc at 5 min, but not at 2 h, after tongue drying on day 7.
Figure 3.
pERK-IR Vc cells on day 7 after tongue drying. Photomicrographs of pERK-IR cells and mean number of pERK-IR cells on day 7 in sham or dry-tongue rats (see Figure 1(a)). (a) Photomicrographs of pERK-IR cells at 5 min after cession of sham treatment. (b) Photomicrographs of pERK-IR cells at 5 min after cession of tongue drying. (c) Mean number of pERK-IR cells at 5 min and 2 h after cession of sham treatment or tongue drying. pERK expression increased at 5 min but not 2 h after cession of tongue drying. (a)a and (b)a: high-magnification photomicrographs of the area indicated by small boxes. Large arrows: the areas pERK-IR cells were observed. Small arrows: pERK-IR cells. **p < 0.01.
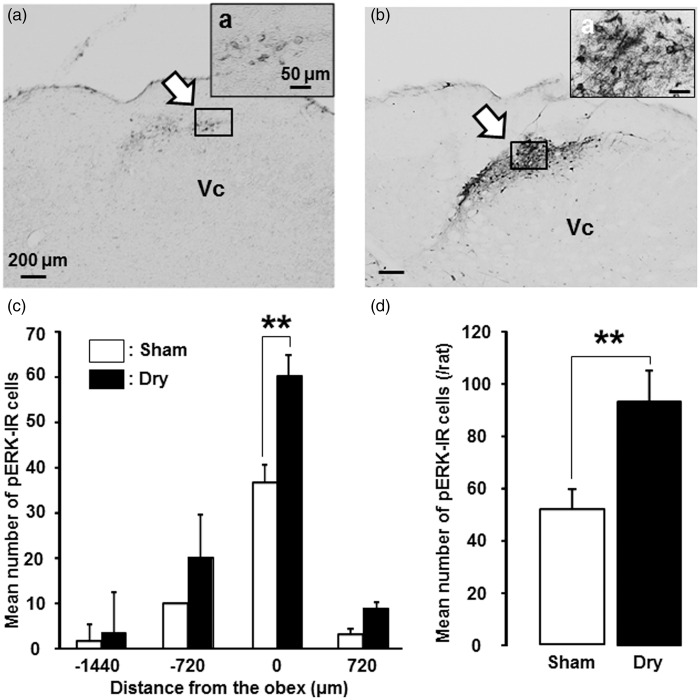
To study ERK phosphorylation related to mechanical stimulation of the tongue, a model of dry-tongue rats at 2 h after secession of tongue-dry treatment were used. Following mechanical stimulation of the tongue, a large number of pERK-IR cells was observed in the dorsal portion of the Vc in dry-tongue rats on day 7, whereas only a small number of them was observed in sham rats on day 7 (Figure 4(a) and (b)). The pERK-IR cells were distributed in the Vc from −1440 µm to +720 µm relative to the obex, and the number of pERK-IR cells was significantly larger in dry-tongue rats compared to sham rats at the obex level (Figure 4(c)). The mean number of pERK-IR cells in the Vc was also significantly larger in dry-tongue rats than sham rats (Figure 4(d)).
Figure 4.
pERK-IR Vc cells after noxious mechanical stimulation in dry-tongue rats. Photomicrographs of pERK-IR cells and mean number of pERK-IR cells following mechanical stimulation of the tongue on day 7 at 2 h after cession of sham treatment or tongue drying (see Figure 1(b)). (a) Photomicrographs of pERK-IR cells in the sham rat. (b) Photomicrographs of pERK-IR cells in the dry-tongue rat. (c) Rostro-caudal distribution of pERK-IR cells in the Vc in sham and dry-tongue rats. (d) Mean number of pERK-IR cells in sham and dry-tongue rats. (a)a and (b)a: high-magnification photomicrographs of the areas indicated by small boxes. Large arrows: the areas pERK-IR cells were observed. **p < 0.01.
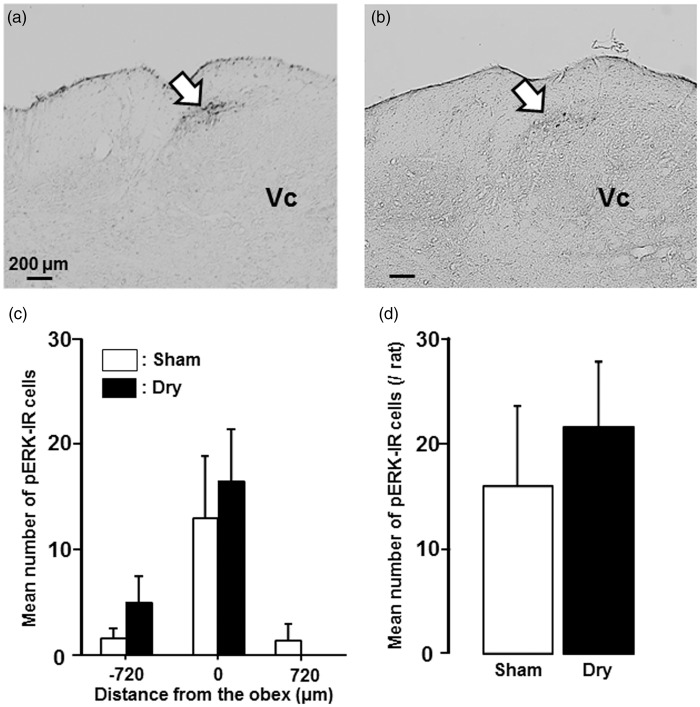
The pERK-IR cells were also observed in the dorsal portion of the Vc following noxious heat stimulation (50℃) of the tongue on day 7 (Figure 5(a) and (b)). These pERK-IR cells were distributed in the Vc from −720 µm to +720 µm relative to the obex (Figure 5(c)), but there were no significant differences in the number of pERK-IR cells following noxious heat stimulation of the tongue between dry-tongue rats and sham rats (Figure 5(c) and (d)).
Figure 5.
pERK-IR Vc cells after noxious heat stimulation in dry-tongue rats. Photomicrographs of pERK-IR cells and mean number of pERK-IR cells following heat stimulation of the tongue on day 7 at 2 h after cession of sham treatment or tongue drying (see Figure 1(b)). (a) Photomicrograph of pERK-IR cells in sham rat. (b) Photomicrograph of pERK-IR cells in dry-tongue rat. (c) Rostro-caudal distribution of pERK-IR cells in the Vc in sham and dry-tongue rats. (d) Mean number of pERK-IR cells in sham and dry-tongue rats. Large arrows: the areas pERK-IR cells were observed.
Effect of PD98059 administration on ERK phosphorylation, nocifensive behavior, and neuronal activity
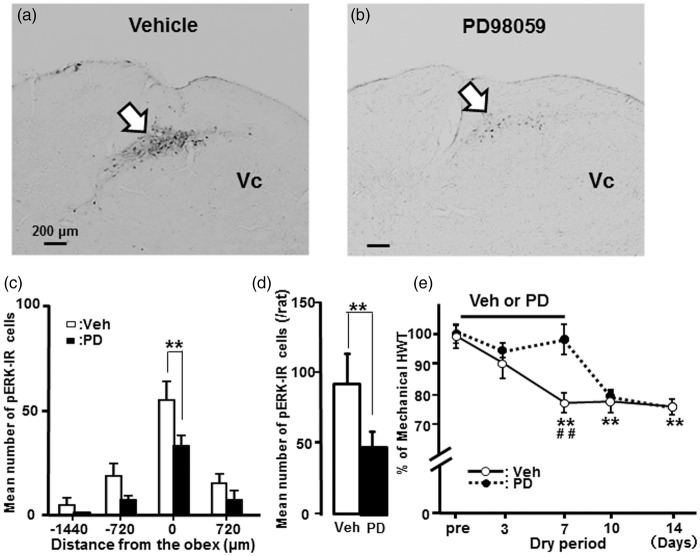
Many pERK-IR cells were observed in the dorsal portion of the Vc following noxious mechanical stimulation of the tongue in vehicle (DMSO)-treated rats after tongue drying on day 7, whereas only a small number were found in MEK1 inhibitor PD98059-treated dry-tongue rats following noxious stimulation of the tongue (Figure 6(a) and (b)). The mean number of pERK-IR cells at the obex level in Vc following noxious mechanical stimulation of the tongue was significantly reduced in PD98059-administrated dry-tongue rats compared to vehicle-treated dry-tongue rats on day 7 (Figure 6(c) and (d)).
Figure 6.
Effect of PD98059 administration on ERK phosphorylation and tongue mechanical hyperalgesia in dry-tongue rats. Photomicrographs of pERK-IR cells and mean number of pERK-IR cells following mechanical stimulation of the tongue on day 7 at 2 h after cession of tongue drying with vehicle of PD90859 administration (see Figure 1(c)). (a) Photomicrograph of pERK-IR cells in vehicle administrated dry-tongue rat. (b) Photomicrograph of pERK-IR cells in PD98059 administrated dry-tongue rat. (c) Rostro-caudal distribution of pERK-IR cells in the Vc in vehicle or PD98059 administrated dry-tongue rat. (d) Mean number of pERK-IR cells in vehicle or PD98059-administrated dry-tongue rat. (e) The effects of vehicle or PD98059 administration on mechanical HWT (percentage) in dry-tongue rats. **p < 0.01 in panel (c) and (d). **p < 0.01 (vs. pre), ##p < 0.01 (Veh vs. PD) in panel (e).
The effect of PD98059 administration on mechanical HWT was also studied in dry-tongue rats. Mechanical HWT was significantly recovered following successive intracisternal (i.c.) administration of PD98059 compared to vehicle administration in dry-tongue rats on day 7 (Figure 6(e)). After the successive i.c. PD98059 administration, HWT values returned to levels similar to those of vehicle-treated dry-tongue rats.
We also studied the effect of PD98059 administration on Vc nociceptive neuronal activities (Figure 7). A total of 46 nociceptive neurons (WDR: n = 35, NS: n = 11), responding to both graded mechanical and heat stimulation of the tongue, were recorded from the Vc in dry tongue and sham rats. All nociceptive neurons were recorded from the dorsal portion of the Vc (Figure 7; Aa: WDR neurons, Ab: NS neurons). The mean BG of Vc nociceptive neurons was not significantly different between sham, vehicle-treated dry-tongue rats and PD98059-treated dry-tongue rats (median [25, 75%], sham: 0.0167 [0.000, 0.517] Hz, n = 15; vehicle in dry tongue: 0.0417 [0.000, 1.850] Hz, n = 18; PD in dry-tongue: 0.208 [0.000, 12.851] Hz, n = 15]. Brush responses of Vc nociceptive neurons were also not significantly different between sham, vehicle-treated dry-tongue, and PD98059-treated dry-tongue rats (Figure 7(b)). Pressure-evoked responses of Vc nociceptive neurons were graded as pressure stimulus intensity was progressively increased, and the mean spike frequency was significantly higher during high-intensity mechanical stimuli (80–150 g) in dry-tongue rats compared to sham rats (Figure 7(c)). Enhanced mechanical-evoked responses were significantly depressed following PD98059 administration compared to vehicle administration in dry-tongue rats (Figure 7(c)). Further, pinch-evoked responses were significantly higher in dry-tongue rats compared to sham rats, and enhanced pinch-evoked responses were significantly recovered to the levels similar to those seen in sham rats following PD98059 administration (Figure 7(d)). On the other hand, we did not observe any significant differences in heat-evoked responses of Vc nociceptive neurons between PD98059-treated rats and vehicle- or sham-treated rats after 7 days tongue drying (Figure 7(e)).
GluR1 phosphorylation in Vc neurons
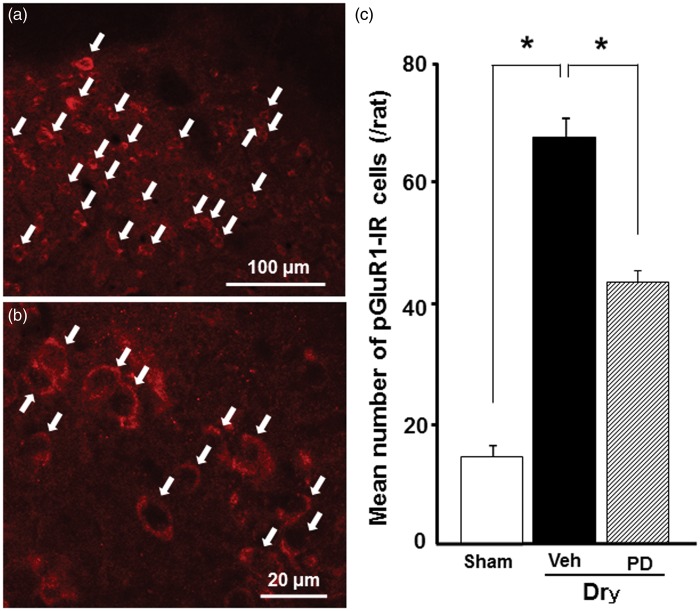
We studied whether GluR1 is phosphorylated in neurons located in dorsal portion of the Vc, where many pERK-IR cells were observed in dry-tongue rats on day 7 (Figure 8(a)). pGluR1-IR products could be seen at the cell body surface, and many pGluR1-IR cells were found in the Vc in vehicle-treated dry-tongue rats on day 7 after tongue drying (Figure 8(b)), whereas a small number of pGluR1-IR cells were observed in the Vc in PD98059-treated dry-tongue rats and sham rats. The mean number of pGluR1-IR cells was significantly larger in dry-tongue rats compared to sham rats and was significantly reduced to the levels similar to those in sham rats on day 7 following PD98059 administration in dry-tongue rats (Figure 8(c)). Furthermore, the mean number of pGulR1-IR cells in the Vc was not significantly different between that of 5 min after tongue drying (67.0 ± 3.0) and that of 5 min after noxious stimulation 2 h after tongue drying (61.0 ± 2.6) on day 7.
Figure 8.
Phosphorylated GluR1 (pGluR1)-IR Vc neurons on day 7 after drying of the tongue. Fluorescent photomicrographs of pGluR1-IR cells (a and b) and mean number of pGluR1-IR cells on day 7 at 5 min after cession of sham treatment, tongue drying with vehicle of PD90859 or PD 98059 administration (see Figure 1(d)). (a) Low magnification photomicrograph of pGluR1-IR cells in dry-tongue rat on day 7. (b) High-magnification photomicrograph of dry-tongue rat on day 7. (c) Mean number of pGluR1-IR cells. Arrows in (a) and (b) indicate pGluR1-IR cells. **p < 0.01.
Discussion
This study provides the first documented evidence of an association of the tongue mechanical (but not heat) hypersensitivity and tongue drying. No obvious inflammation was observed in the tongue following the treatment of tongue drying (data not shown). Without any stimulus, the number of pERK-IR cells in the Vc was significantly larger 5 min after cessation of the tongue dry in dry-tongue rats compared to sham rats. Following noxious mechanical, but not heat, stimulation of the tongue, the number of pERK-IR cells was also significantly larger in dry-tongue rats compared to sham rats. Mechanical-evoked responses of Vc nociceptive neurons were significantly larger in dry-tongue rats compared to sham rats, whereas heat-evoked responses were not altered after tongue drying. The number of pGluR1-IR cells was also significantly larger in dry-tongue rats compared to sham rats. The number of pGluR1-IR cells was significantly decreased following i.c. administration of PD98059 but not by vehicle administration, indicating the involvement of pERK in GluR1 phosphorylation in Vc nociceptive neurons. Furthermore, the number of pERK-IR cells 2 h after secession of tongue drying and spontaneous activity of Vc nociceptive neurons were not larger in dry-tongue rats compared to sham rats.
Enhancement of mechanical nocifensive reflex following tongue drying
The rat model of dry tongue was developed by drying the rat’s tongue for 7–14 days. In the dry-tongue rats, tongue hypersensitivity to mechanical, but not heat, stimulation was produced on days 7–14. It is unclear why mechanical hypersensitivity but not heat hypersensitivity occurs following drying of the tongue. Receptor mechanisms in the primary afferent neurons are thought to be involved in tongue mechanical hypersensitivity associated with tongue drying. It is well known that the transient receptor potential (TRP) channels are involved in heat, cold, and mechanical sensitivity of the primary afferent neurons.23 These channels are sensitized under pathological conditions such as peripheral inflammation or peripheral nerve injury and become hypersensitive to a variety of noxious and/or non-noxious stimuli. It may be probable that TRP channel mechanisms are involved in tongue mechanical hypersensitivity associated with tongue drying. Further studies are needed to clarify the basis of differential hypersensitivity in the primary afferent neurons.
Sensitization of Vc nociceptive neurons
A number of previous studies have reported that the ERK is phosphorylated in SDH or Vc and C1-C2 neurons following various noxious stimuli to the orofacial region.24,25 ERK phosphorylation occurs within 10 min, and the number of pERK-IR cells increases as noxious stimulus intensity increases.24 Further, it has been reported that ERK phosphorylation in dorsal horn neurons is suppressed associated with NMDA receptor blockade, indicating that NMDA receptor has an important role in ERK phosphorylation.26 ERK phosphorylation has also been reported to be involved in wind-up phenomena of dorsal horn nociceptive neurons.12 These findings indicate that ERK phosphorylation in second-order neurons is a good indicator of activation of nociceptive neurons. We observed many pERK-IR cells 5 min after noxious mechanical, but not heat, stimulation of the dried tongue on day 7, and these cells were restricted in the dorsal portion of the Vc. The pERK-IR cells in the Vc reported in previous studies had round soma and many fibers, and most of them also showed NeuN-IR.10 Since pERK-IR cells we observed in this study had similar features to those reported in the previous one, pERK-IR cells in this study were defined as neurons.
It is well known that the Vc is subdivided into three portions, ventral, middle, and dorsal portions, according to the innervation areas of trigeminal nerve branches (ventral: third branch, middle: second branch and dorsal : first branch).27 The dorsal portion of the Vc, where many pERK-IR cells were observed following tongue drying, is referred to as the third branch region according to the previous anatomical and electrophysiology studies, suggesting that the dorsal portion of the Vc is the area receiving noxious inputs from the tongue.24 Furthermore, we observed a significantly larger number of pERK-IR cells in the dorsal portion of the Vc in dry-tongue rats compared to sham rats following mechanical stimulation of the tongue. In fact, the reduced HWT to mechanical stimulation of the tongue was significantly recovered following i.c. administration of PD98059 in dry-tongue rats. These data suggest that the pERK-IR cells in the dorsal portion of the Vc are sensitized by tongue drying, resulting tongue mechanical, but not heat, hypersensitivity.
Based on our neuron recording experiment, the Vc nociceptive neurons were classified as WDR and NS neurons, and non-noxious mechanical and heat responses of these nociceptive neurons were not enhanced in dry-tongue rats. Nonetheless, the noxious pressure and pinch responses of these neurons were significantly enhanced in dry-tongue rats compared to sham rats. These suggest that the high-threshold mechanical primary afferent fibers might be sensitized in dry-tongue rats, resulting in the enhancement of noxious pressure and pinch responses of Vc nociceptive neurons. Indeed, the enhancement of high-threshold mechanical responses was significantly suppressed by PD98059 administration in dry-tongue rats compared to vehicle-administrated rats, suggesting that ERK phosphorylation is actively involved in the enhancement of high-threshold mechanical responses of Vc nociceptive neurons in dry-tongue rats.
Involvement of GluR1 phosphorylation in Vc neuron sensitization
The pGluR1-containing AMPAR is known to be involved in the modulation of neuronal excitability in the Vc under the pathological states.28 It has also been reported that the GluR1 phosphorylation is involved in sensitization of nociceptive neurons in SDH following peripheral nerve injury or inflammation.15 The blockade of GluR1 phosphorylation, which is produced by noxious stimulation in SDH, induces the reduction of both, Fos expression and nocifensive behavior, in rats with inflammation of the paw.29 The pGluR1 is also known to be involved in AMPAR membrane trafficking via calcium/calmodulin-dependent protein kinase II cascade associated with NMDAR activation.30 The blockade of NMDAR in ERK phosphorylation, caused by noxious stimulation of the foot, was reported being reduced in SDH neurons, suggesting that the NMDAR is a key receptor involved in ERK phosphorylation in SDH neurons.26 These findings suggest that pGluR1 and ERK phosphorylation occurs following NMDAR activation in dry-tongue rats.
We also observed a significant increase in the number of pGluR1-IR cells in the Vc at 7 days after tongue drying. Furthermore, significant reduction of the number of pGluR1-IR cells was produced by i.c. administration of PD98059. Noxious mechanical but not heat-evoked responses of Vc nociceptive neurons were significantly enhanced in dry-tongue rats yet were significantly diminished following i.c. administration of PD98059. The previously cited articles including our study results suggest that the ERK phosphorylation in fact causes an enhancement of pGluR1 phosphorylation in AMPAR, resulting in an increase in the noxious mechanical evoked response of Vc nociceptive neurons in dry-tongue rats.
Conclusion
The present findings are summarized in Figure 9. The data suggest that after drying of the tongue in rats, the GluR1 phosphorylation is enhanced in association with ERK phosphorylation in Vc neurons, thus inducing the enhancement of mechanical responses of the Vc WDR and NS neurons. To conclude, the pERK-pGluR1 cascade in Vc nociceptive neurons is involved in tongue mechanical hypersensitivity associated with dry mouth. On the other hand, the number of pERK-IR cells 2 h after secession of tongue drying and spontaneous activity of Vc nociceptive neurons were not larger in dry-tongue rats compared with sham rats, suggesting that the pERK-pGluR1 cascade in Vc nociceptive neurons may not be involved in modulation of BG of Vc nociceptive neurons.
Figure 9.
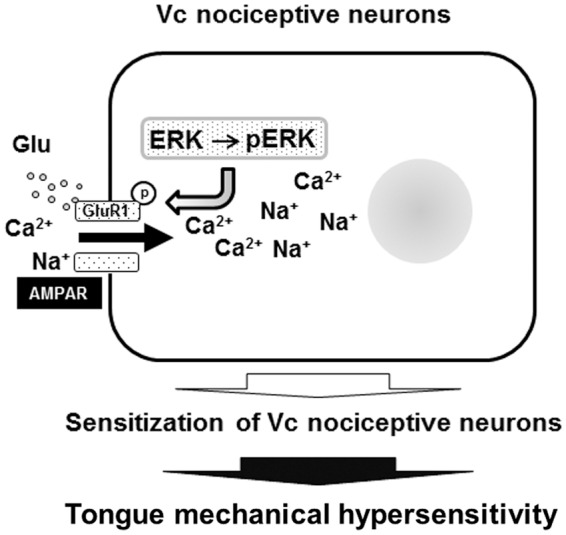
Schematic illustration of ERK-GluR1 cascade in Vc nociceptive neurons in dry tongue rats. GluR1 is phosphorylated in association with ERK phosphorylation, and then spike generation is enhanced in Vc nociceptive neurons, resulting in central sensitization and tongue mechanical hypersensitivity.
Author Contributions
YN, YT, AO, AK, JYC, NN, and DB performed animal experiments, immunohistochemistry, and pharmacological testing, and analyzed the data; MS, YI, BJS, and KI designed experiments, supervised research, and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by Research grants from Sato and Uemura Funds from Nihon University School of Dentistry, and Grant from Dental Research Center, Nihon University School of Dentistry; Nihon University multidisciplinary research grant and Individual Research Grant; KAKENHI (Grant-in-Aid for Scientific Research (C) (#26460708, #20362238, #25463151, #15K11324, # 15K11272); grants from MEXT-Supported Program for the Strategic Research Foundation at Private Universities 2013–2017.
References
- 1.Kramer JM. Current concepts in Sjogren’s syndrome and considerations for the dental practitioner. NY State Dent J 2015; 81: 24–29. [PubMed] [Google Scholar]
- 2.Casterline PF, Jaques DA. The surgical management of recurrent parotitis. Surg Gynecol Obstet 1978; 146: 419–422. [PubMed] [Google Scholar]
- 3.Aggarwal H, Pal-Singh M, Mathur H, et al. Evaluation of the effect of transcutaneous electrical nerve stimulation (TENS) on whole salivary flow rate. J Clin Exp Dent 2015; 7: e13–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field A, Longman L, Tyldesley WR. Tyldesley’s oral medicine, 5th ed London: Oxford University Press, 2003. [Google Scholar]
- 5.Story GM, Peier AM, Reeve AJ, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003; 112: 819–829. [DOI] [PubMed] [Google Scholar]
- 6.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol 2011; 97: 179–206. . [DOI] [PubMed] [Google Scholar]
- 7.Shinoda M, Asano M, Omagari D, et al. Nerve growth factor contribution via transient receptor potential vanilloid 1 to ectopic orofacial pain. J Neurosci 2011; 31: 7145–7155. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiyomoto M, Shinoda M, Okada-Ogawa A, et al. Fractalkine signaling in microglia contributes to ectopic orofacial pain following trapezius muscle inflammation. J Neurosci 2013; 33: 7667–7680. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu K, Matsumoto K, Noma N, et al. Involvement of trigeminal transition zone and laminated subnucleus caudalis in masseter muscle hypersensitivity associated with tooth inflammation. PloS One 2014; 9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki I, Tsuboi Y, Shinoda M, et al. Involvement of ERK phosphorylation of trigeminal spinal subnucleus caudalis neurons in thermal hypersensitivity in rats with infraorbital nerve injury. PloS One 2013; 8: e57278. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda K, Kitagawa J, Sessle BJ, et al. Mechanisms involved in an increment of multimodal excitability of medullary and upper cervical dorsal horn neurons following cutaneous capsaicin treatment. Mol Pain 2008; 4: 59. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukui T, Dai Y, Iwata K, et al. Frequency-dependent ERK phosphorylation in spinal neurons by electric stimulation of the sciatic nerve and the role in electrophysiological activity. Mol Pain 2007; 3: 18. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishina M, Sakimura K, Mori H, et al. A single amino acid residue determines the Ca2+ permeability of AMPA-selective glutamate receptor channels. Biochem Biophys Res Commun 1991; 180: 813–821. [DOI] [PubMed] [Google Scholar]
- 14.Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science 1991; 252: 851–853. [DOI] [PubMed] [Google Scholar]
- 15.Wang JQ, Guo ML, Jin DZ, et al. Roles of subunit phosphorylation in regulating glutamate receptor function. Eur J Pharmacol 2014; 728: 183–187. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn SM, Choe ES. Activation of group I metabotropic glutamate receptors increases serine phosphorylation of GluR1 alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in the rat dorsal striatum. J Pharmacol Exp Therapeut 2009; 329: 1117–1126. . [DOI] [PubMed] [Google Scholar]
- 17.Boehm J, Kang MG, Johnson RC, et al. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron 2006; 51: 213–225. . [DOI] [PubMed] [Google Scholar]
- 18.Lee HK, Takamiya K, Han JS, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 2003; 112: 631–643. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 20.Saito K, Hitomi S, Suzuki I, et al. Modulation of trigeminal spinal subnucleus caudalis neuronal activity following regeneration of transected inferior alveolar nerve in rats. J Neurophysiol 2008; 99: 2251–2263. . [DOI] [PubMed] [Google Scholar]
- 21.Katagiri A, Shinoda M, Honda K, et al. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol Pain 2012; 8: 23. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda K, Shinoda M, Furukawa A, et al. TRPA1 contributes to capsaicin-induced facial cold hyperalgesia in rats. Eur Journal Oral Sci 2014; 122: 391–396. . [DOI] [PubMed] [Google Scholar]
- 23.Yin K, Zimmermann K, Vetter I, et al. Therapeutic opportunities for targeting cold pain pathways. Biochem Pharmacol 2015; 93: 125–140. . [DOI] [PubMed] [Google Scholar]
- 24.Noma N, Tsuboi Y, Kondo M, et al. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J Comp Neurol 2008; 507: 1428–1440. . [DOI] [PubMed] [Google Scholar]
- 25.Tsujimura T, Shinoda M, Honda K, et al. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis, upper cervical cord, NTS and Pa5 following capsaicin injection into masticatory and swallowing-related muscles in rats. Brain Res 2011; 1417: 45–54. . [DOI] [PubMed] [Google Scholar]
- 26.Ji RR, Baba H, Brenner GJ, et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999; 2: 1114–1119. . [DOI] [PubMed] [Google Scholar]
- 27.Shibuta K, Suzuki I, Shinoda M, et al. Organization of hyperactive microglial cells in trigeminal spinal subnucleus caudalis and upper cervical spinal cord associated with orofacial neuropathic pain. Brain Res 2012; 1451: 74–86. . [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto M, Tsuboi Y, Honda K, et al. Involvement of AMPA receptor GluR2 and GluR3 trafficking in trigeminal spinal subnucleus caudalis and C1/C2 neurons in acute-facial inflammatory pain. PloS One 2012; 7: e44055. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pezet S, Marchand F, D’Mello R, et al. Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions. J Neurosci 2008; 28: 4261–4270. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakegawa W, Tsuzuki K, Yoshida Y, et al. Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses. Eur J Neurosci 2004; 20: 101–110. . [DOI] [PubMed] [Google Scholar]