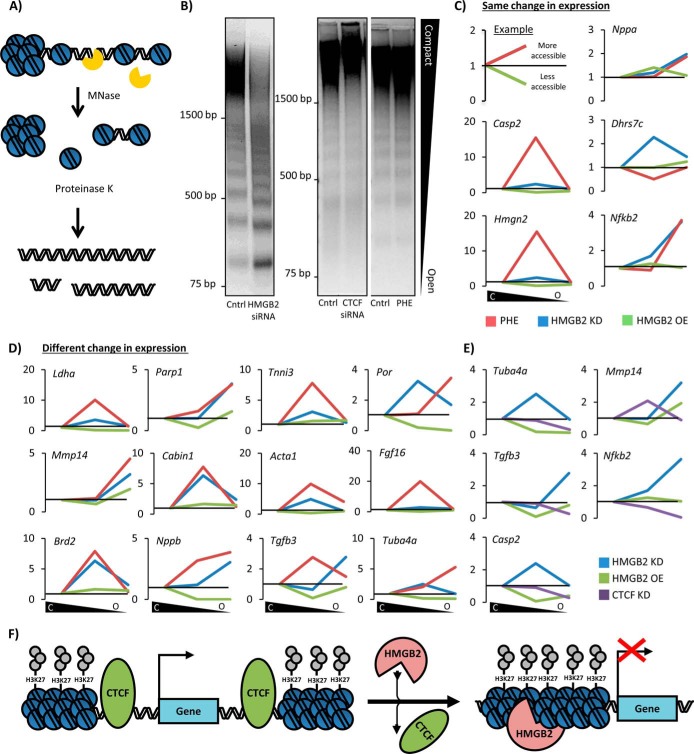
FIGURE 7.
Hmgb2 and Ctcf control gene accessibility in an antithetic manner at the level of the chromatin fiber. A, chromatinized genomic DNA was treated with MNase (0.001 units), resulting in partial digestion of the genome based on accessibility. B, hmgb2 knockdown increased the abundance of highly digested lower molecular weight fragments of DNA (representing open chromatin), while decreasing the abundance of less digested higher molecular weight fragments (compact chromatin), suggesting a global increase in DNA accessibility. Such dramatic change in global accessibility occurred with neither ctcf knockdown nor phenylephrine treatment (one representative experiment of 6). C, after MNase digestion, DNA fragments were cut from the compact, intermediate, and open regions of the agarose gel and analyzed by qPCR to determine the relative distribution of individual promoter sequences for mRNA-coding genes of interest. Plotted is the change in the ratio of open chromatin to heterochromatin, and intermediate chromatin to heterochromatin, between control and treated cells; as shown in the 1st panel, these ratios are represented in the ensuing graphs as upward inflections for a shift to more accessible chromatin by a given gene and downward deflections for a gene that shifts to more compact DNA. Gene expressions for hmgb2 knockdown and phenylephrine from microarray data in NRVMs (21) were used to distinguish between genes with similar expression changes induced by hmgb2 and phenylephrine (C) or genes regulated differently by hmgb2 and phenylephrine (D). Interestingly, hmgb2 knockdown and phenylephrine showed similar trends for shifting promoter sequences between these categories even when these stimuli (hmgb2 knockdown or phenylephrine) had different effects on the transcription of the gene. E, promoter sequences for the genes bound by Ctcf and Hmgb2 by ChIP were also examined. In four of the five cases, ctcf knockdown shifted these sequences to more compact regions of chromatin. (All MNase data are the average of at least three experiments.) F, model for relationship between Hmgb2 and Ctcf. Ctcf serves as a boundary preventing the spread of heterochromatin, whereas Hmgb2 promotes heterochromatin formation.