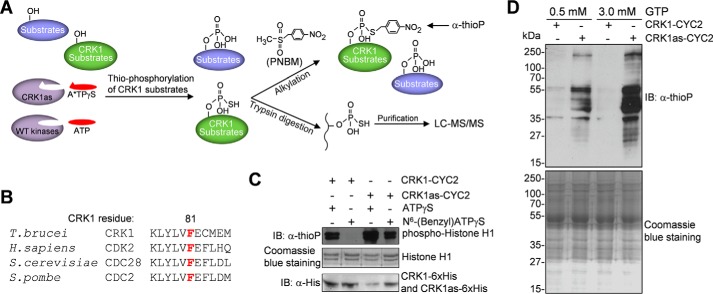
FIGURE 1.
Identification of CRK1 substrates by chemical genetic approach. A, procedures of the chemical genetic approach used for CRK1 substrate detection and identification. Thiophosphorylated CRK1 substrates were either detected by Western blotting after PNBM alkylation, which yields thiophosphate ester and can be detected by a thiophosphate ester-specific rabbit monoclonal antibody (anti-thioP), or trypsin-digested, affinity-purified, and analyzed by LC-MS/MS. B, alignment of the sequence surrounding the gatekeeper residue of CRK1 with its orthologs from human, budding yeast, and fission yeast. The gatekeeper residue is highlighted in red. C, in vitro kinase assays to confirm that the CRK1as, but not the wild-type CRK1, is capable of utilizing the bulky ATP analog to thiophosphorylate histone H1. CRK1 (wild-type and analog-sensitive mutant) and CYC2 were co-expressed in E. coli and co-purified. Thiophosphorylated histone H1 was detected by Western blotting with a thiophosphate ester monoclonal antibody (α-thioP). CRK1-His6 and CRK1as-His6 were detected with anti-His antibody, whereas histone H1 was detected by Coomassie Blue staining. D, in vitro thiophosphorylation of T. brucei total proteins by CRK1as. Crude trypanosome cell lysate was incubated with purified CRK1-CYC2 complex and CRK1as-CYC2 complex in the presence of N6-(benzyl)ATPγS. GTP (0.5 or 3 mm) was added to the kinase reaction to act as a competitor of nonspecific labeling. Thiophosphorylated proteins were detected by Western blotting with α-thioP antibody. Total T. brucei proteins used for in vitro kinase assay were stained with Coomassie Blue. IB, immunoblot.