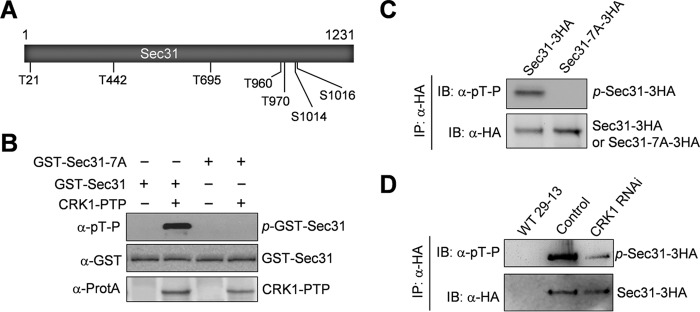
FIGURE 2.
Sec31 is phosphorylated by CRK1 at multiple serine and threonine residues. A, schematic drawing of the Sec31 protein, showing the seven serine and threonine residues that are phosphorylated in vivo and are putative CRK1 phosphosites. B, phosphorylation of Sec31 by CRK1 in vitro. Sec31 and Sec31-7A were purified as GST fusion protein from E. coli. CRK1-PTP was immunoprecipitated from trypanosome cell lysate. Phosphorylated GST-Sec31 (p-GST-Sec31) was detected by Western blotting with anti-phosphothreonine-proline (α-pT-P) antibody. GST-Sec31 was detected with anti-GST antibody, whereas CRK1-PTP was detected with anti-protein A antibody (α-ProtA). C, confirmation of in vivo Sec31 phosphorylation by Western blotting with anti-phosphothreonine-proline antibody. Sec31 and Sec31-7A were overexpressed in T. brucei, immunoprecipitated (IP) with anti-HA antibody, and then immunoblotted (IB) with anti-phosphothreonine-proline (α-pT-P) antibody to detect the phosphorylated serine and threonine residues followed by a proline in Sec31–3HA and with anti-HA antibody to detect 3HA-tagged Sec31 and Sec31-7A. D, depletion of CRK1 caused dephosphorylation of Sec31 in T. brucei. Sec31 was endogenously tagged with a C-terminal triple HA epitope in the T. brucei cell line harboring the CRK1 RNAi construct. RNAi was induced for 2 days. Sec31–3HA was immunoprecipitated from non-induced control and CRK1 RNAi cells, and immunoblotted with anti-phosphothreonine-proline (α-pT-P) antibody to detect phosphorylated Sec31–3HA and with anti-HA antibody to detect Sec31–3HA. The wild-type 29-13 cell line served as a mock control.