FIGURE 1.
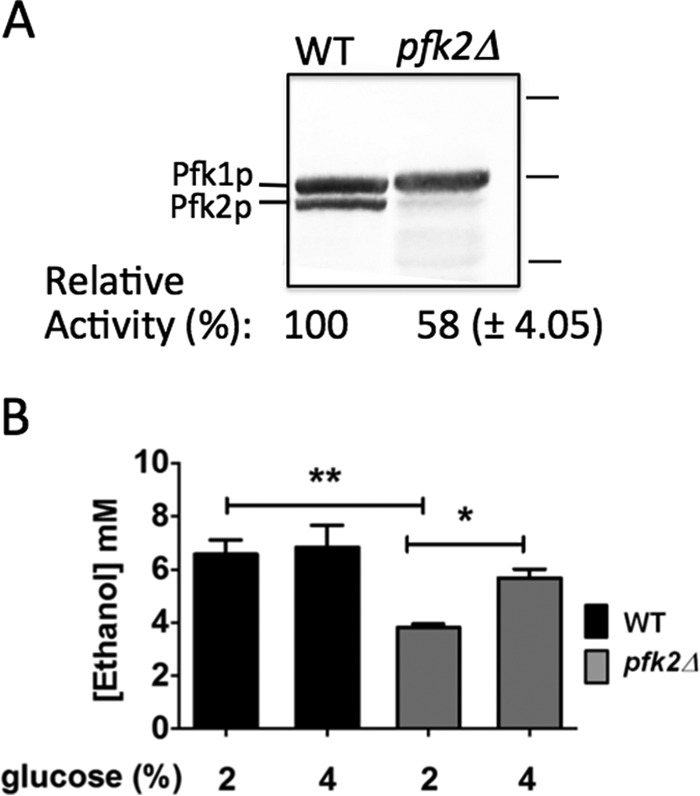
PFK-1 activity and glycolysis are defective in pfk2Δ cells. A, PFK-1 enzymatic activity is reduced in pfk2Δ cells. Wild-type and pfk2Δ cells were grown overnight to mid-log phase (optical density of 0.6–0.8 A600/ml) in YEP medium containing 2% glucose. The cells were converted to spheroplast by zymolase treatment, and protein was separated on a 10% SDS-PAGE gel. The gel was immunoblotted with primary polyclonal antibodies to PFK-1 and secondary antibodies conjugated to alkaline phosphate. Protein markers (right) are 150, 100, and 75 kDa. PFK-1 enzymatic activity was measured for an optical density of 20.0 A600 obtained from cells grown overnight to mid-log phase in medium containing 2% glucose. Data are expressed as the average ± S.D. (n = 3 separate experiments). Relative averaged values were used to express percentage activity. The wild-type PFK-1 activity in the whole cell lysate was 0.014–0.017 μmol of fructose 1,6-bisphosphate/min/optical density A600. B, ethanol concentration is lower in pfk2Δ than in wild-type cells. The cells were cultured to an optical density of 0.6–0.8 A600/ml in YEP medium containing 2% or 4% glucose, converted to spheroplasts by zymolase treatment, and resuspended in fresh medium containing 1.2 m sorbitol plus 2% or 4% glucose. After 20 min at 30 °C, the ethanol concentration was measured. Data represent three independent experiments. Error bars are standard deviation. Statistically significant differences (*, p < 0.05; **, p < 0.01) were determined by two-tailed unpaired t test.
