FIGURE 5.
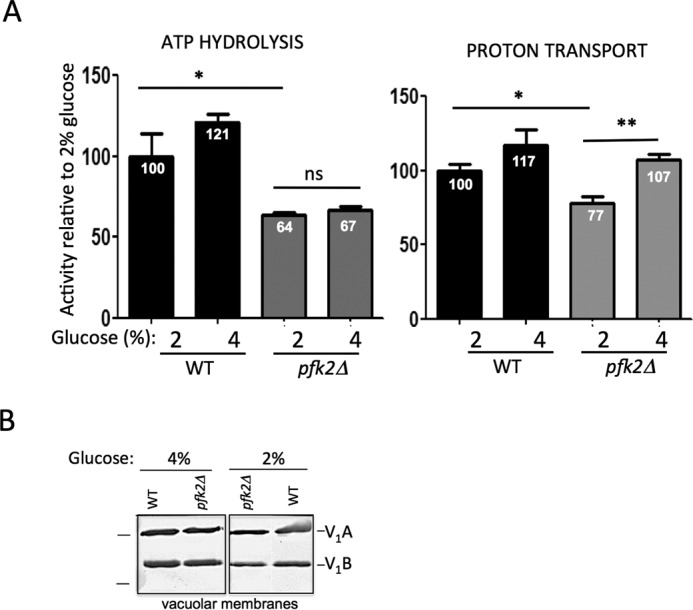
Proton transport is increased at vacuolar membranes from pfk2Δ cells cultured in 4% glucose. A, V-ATPase coupling efficiency is increased in 4% glucose. Vacuolar membrane fractions from wild-type and pfk2Δ cells were purified by density centrifugation. ATP hydrolysis (left panel) was assayed spectrophotometrically in the presence and absence of the V-ATPase inhibitor concanamycin A (100 nm) by using an enzymatic coupled assay that measures NADH oxidation at 340 nm. The average wild-type specific activity in 2% glucose for the concanamycin A-sensitive ATP hydrolysis was 3.0 μmol of ATP/min/mg of total vacuolar protein. ATP-dependent proton transport (right panel) was measured by fluorescence quenching of 1 μm 9-amino-6-chloro-2-methoxyacridin (excitation, 410 nm; emission, 490 nm) upon addition of 0.5 mm ATP/1 mm MgSO4 to 5 μg of total protein in vacuolar membrane vesicles. Initial velocities were calculated for 15 s following MgATP addition. The average wild-type slope was −1117.47 fluorescence units/15 s. Data represent six independent vacuolar preps. Statistically significant differences (*, p < 0.05; **, p < 0.01; ns, not significant) were as compared with the wild type in the presence of 2% glucose and determined by two-tailed unpaired t test. B, V-ATPase assembly is comparable in 4% glucose and 2% glucose. Vacuolar membrane vesicles were purified from pfk2Δ and wild-type cells cultured overnight in 2% glucose or 4% glucose. Membrane protein (1 μg total membrane protein/well) was separated by SDS-PAGE in 10% gels. Gels were immunoblotted with primary monoclonal antibodies to V1 subunit A and subunit B, and secondary antibodies conjugated to alkaline phosphate. Protein markers (left) are 77 and 50 kDa. This gel was modified to excise a lane containing pfk1Δ membranes.
