FIGURE 5.
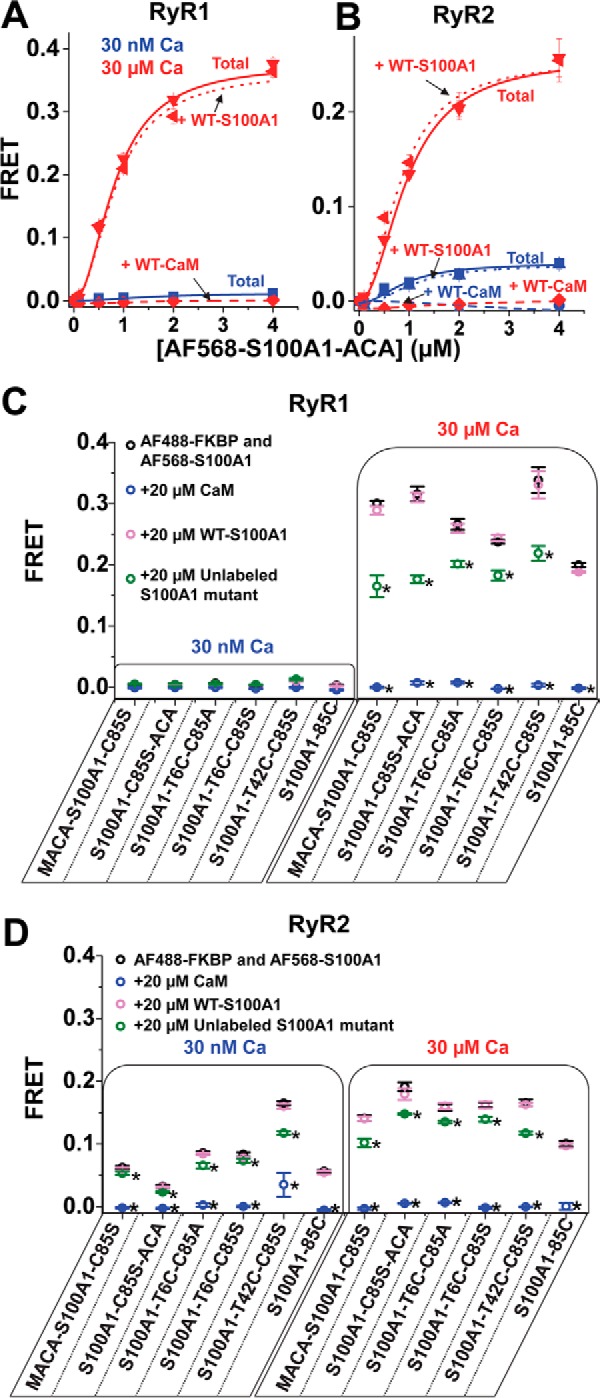
Competition of A-S100A1 by unlabeled S100A1 and CaM for binding to RyR. RyRs in SR membranes isolated from skeletal or cardiac muscle were labeled with D-FKBP and incubated with A-S100A1. Binding specificity of A-S100A1 to RyR was investigated by the addition of a >10-fold molar excess of unlabeled S100A1 or CaM. Saturation binding of AF568-S100A1-C85S-ACA to RyR from skeletal (A) and cardiac (B) SR is indicated as Total (solid lines) at 30 nm Ca2+ (blue) and 30 μm Ca2+ (red). Total FRET is compared with the saturation binding curve in the presence of 40 μm WT-S100A1 (dotted lines) or 40 μm WT-CaM (dashed lines). Binding specificities of several A-S100A1 variants to RyR from skeletal SR (C) or cardiac SR (D) were tested by the addition of 20 μm CaM (blue circles), WT-S100A1 (pink circles), or unlabeled S100A1 (green circles). Data are expressed as means ± S.E. (n = 4). *, significantly different from respective control (black circles), p < 0.05, as determined by analysis of variance with Fisher's post hoc test.
