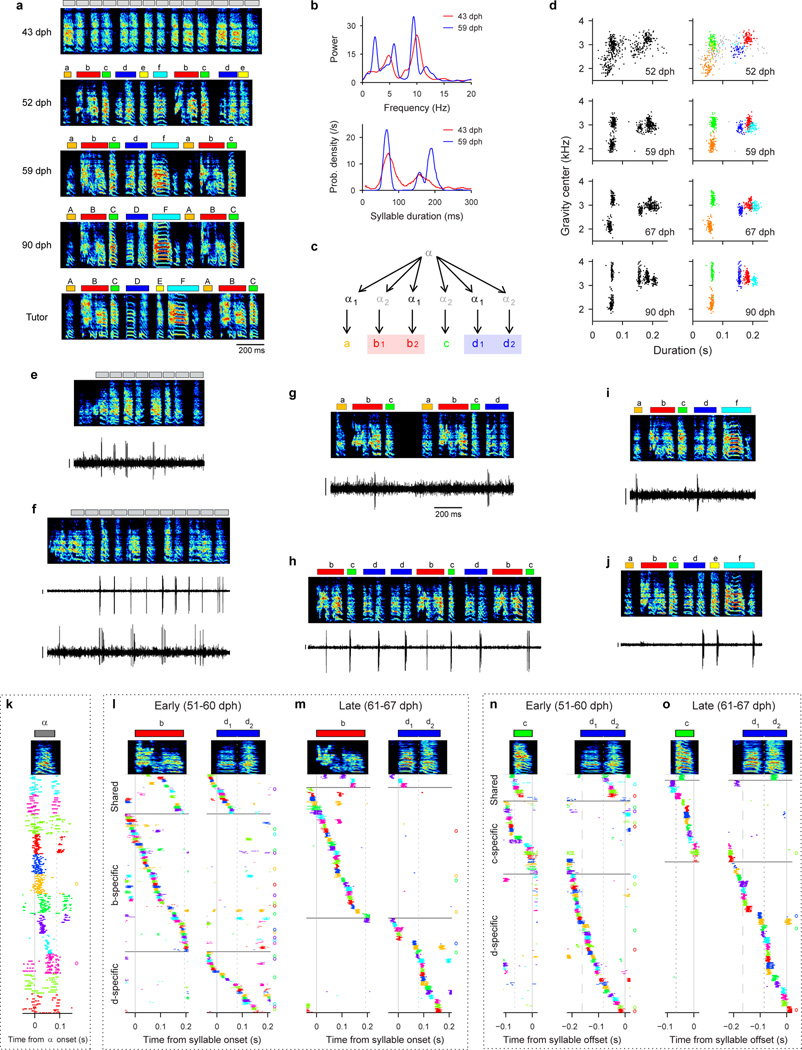
Extended Data Figure 9. Another example of shared burst sequences during the emergence of new syllable types.
All data are from Bird 4. a, Song examples during the emergence of a motif ABCDF. Note the nearly simultaneous emergence of multiple syllable types in fixed order (52 dph). Tutor song shown at the bottom. Phase segments are shown above the spectrogram for song at 43 dph. b, Top: Song rhythm spectrum calculated in the protosyllable stage (43 dph) and after motif formation (59 dph). Note the pronounced peaks at 5 Hz and 10 Hz in both stages. Bottom: Syllable duration distribution in the protosyllable stage (43 dph) and after motif formation (59 dph) showing two peaks. At 43 dph, the peak at 70 ms indicates short protosyllables corresponding to one cycle of the 10 Hz rhythm, and the peak at 140 ms indicates longer syllables formed by two protosyllables fused across two cycles of the 10 Hz rhythm (doubled protosyllables). Example doubled protosyllables are seen in the first and third syllables of panel a (43 dph; note that boxes at the top of this panel indicate phase segments, not syllable boundaries). c, Hypothesized mechanism of motif construction, based on the examination of acoustic structure and analysis of neural burst sequences (see below). Notably, in this bird, the majority of syllables emerged nearly simultaneously in a relatively fixed order, consistent with a ‘motif strategy.’ d, Scatter plots of syllable duration versus mean spectral center of gravity at four stages of vocal development (each dot represents a single syllable; n=500 syllables per day; color coded according to syllable identity in panel a). e, Neuron bursting at 10 Hz protosyllable rhythm (HVCX; 48 dph). Phase segments shown above spectrogram. f, Top: neuron bursting at the 10 Hz rhythm (HVCX; 49 dph). Bottom: Simultaneous recording of a neuron bursting on alternate cycles of 10 Hz rhythm (HVCRA). g, Shared neuron bursting on second half of syllable ‘b’ (labeled b2) and first half of syllable ‘d’ (labeled d1) (HVCRA; 51 dph). h, Shared neuron bursting rhythmically on first half of ‘b’ (b1), syllable ‘c’ and second half of ‘d’ (d2) (HVCRA; 51 dph). i, Shared neuron bursting on ‘a’ and first half of ‘d’ (d1) (HVCRA; 58 dph). j, Shared neuron bursting on second half of ‘d’ (d2), ‘e’, and last part of ‘f’ (HVCRA; 57 dph). k, Population raster of 12 neurons that were significantly locked to protosyllable onsets (48–49 dph). Protosyllables were identified using phase segmentation (see Methods). l, Population raster showing neurons active during syllables ‘b’ and/or ‘d’, recorded early in syllable differentiation. Neurons shared between ‘b’ and ‘d1’ are grouped at top. Neurons specific for ‘b’ are grouped next, and neurons specific for ‘d’ are grouped at bottom. m, Same as panel l but for neurons recorded later in development. n, Population rasters showing neurons active during syllables ‘c’ and/or ‘d’, recorded early in development. o, Same as panel m, but for neurons recorded later in development. Scale bars: (e–j) 0.5 mV, 200 ms.
Neural evidence for hypothesized mechanism of motif construction. Based on an analysis of acoustic signals and neural recordings, we have formulated a hypothesis for how the song of this bird developed, from the formation of the protosyllable to the emergence of the complete motif. We hypothesize that the fundamental protosyllable element corresponds to the prominent 10 Hz peak in the rhythm spectrum and the 70 ms peak in the duration distribution (panel b). This view is further supported by the presence of neurons in the protosyllable stage that generate rhythmic bursts at 10 Hz (panels e, f; 11/18 neurons were rhythmic, 5/11 rhythmic neurons exhibited periodicity at 10 Hz), and the existence of a burst sequence during the protosyllable (panel k).
In this bird, the rhythmic protosyllables differentiated nearly simultaneously, at an early age (52 dph, panel a), into a complete sequence of distinct syllables that subsequently formed the adult song, suggesting this bird employed a ‘motif strategy.’ One complication of this simple view is that there may have been an early partial splitting of the short protosyllable α into two ‘daughter’ protosyllables α1 and α2, which alternated to produce the elements of the final motif (panel c). Two lines of evidence based on neural activity support this view: First, many neurons recorded at an early stage (<50 dph) exhibited a prominent 5 Hz periodicity in their rhythmic bursting. (panels f, h; 6/11 rhythmic neurons), rather than the expected 10 Hz period (panel e, f, top). This observation led us to consider the possibility that the 100 ms neural sequence, corresponding to the dominant 10 Hz protosyllable rhythm, underwent a partial splitting during the protosyllable stage—similar to the alternating differentiation described for Bird 1 (Fig. 3; Extended Data Fig. 4). This would result in two distinct alternating protosyllable sequences α1 and α2 (panel c). Such splitting would effectively double the period of the protosyllable rhythm, and would account for the ‘doubled’ protosyllables and the 5 Hz peak in the rhythm spectrum (panel b).
The existence of short and doubled protosyllables led us to hypothesize that the short syllables of the adult motif (‘a’, ‘c’, and ‘e’) arose from the short protosyllables, while long adult syllables (‘b’ and ‘d’, and possibly ‘f’) arose from the doubled protosyllables (panel c). Early syllable ‘e’ is later dropped by the juvenile, although it appears in the tutor song.
Furthermore, the analysis of shared sequences (panels l–o) revealed a predominance of shared neurons between syllable elements in alternating cycles of the underlying 10 Hz rhythm. For example, shared neurons were observed between syllables ‘a’, ‘b2’ and ‘d1’ (panel i for neuron shared between ‘a’ and ‘d1’; panel g and l for neurons shared between ‘b2’ and ‘d1’). Shared neurons were also observed between syllables ‘b1’, ‘c’, and ‘d2’ (panel h for neuron shared between ‘b1’, ‘c’, and ‘d2’; panel n for neurons shared between ‘c’ and ‘d2’). In contrast, many fewer shared neurons were observed between neighboring cycles of the underlying rhythm, although examples of this can be found (panel j).