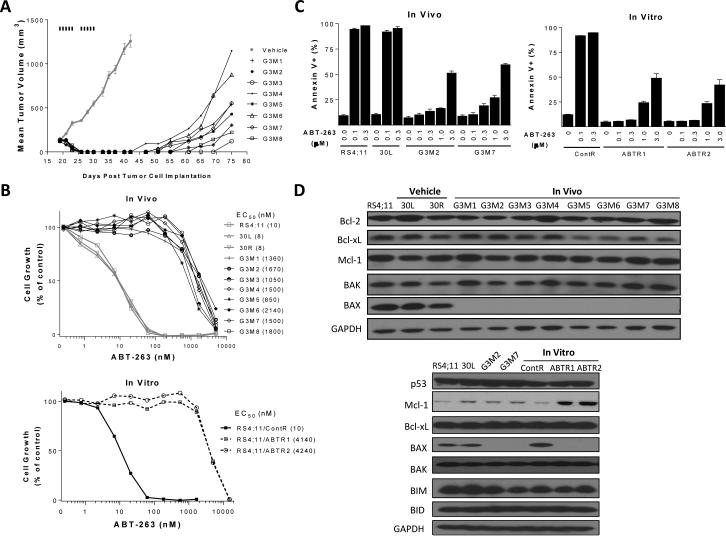
Figure 1.
Establishment and characterization of in vitro and in vivo RS4;11 sublines resistant to ABT-263/ABT-737. A, Establishment of in vivo RS4;11 sublines. RS4;11 xenograft tumors were treated with vehicle or ABT-737 at 100 mg/kg, i.p. for 5 days per week for 2 weeks. Vehicle treated tumors and ABT-737-treated, regrown tumors were harvested and cultured to generate sublines. B, Sensitivity of in vivo and in vitro RS4;11 sublines to ABT-263 in a cell growth assay. Parental, two representative vehicle-treated sublines (30L and 30R) and 8 sublines (G3M1-G3M8) generated from ABT-treated tumors were treated with ABT-263 or vehicle control for 4 days and cell viability was determined by a WST assay. C, Sensitivity of in vivo and in vitro RS4;11 sublines to ABT-263 in an apoptosis assay. Parental RS4;11 cell line and sublines established from RS4;11 xenografts (30L, vehicle-treated; G3M2 and G3M7, SAR405838-treated) and RS4;11 sublines established in vitro (ContR, vehicle-treated; ABTR1 and ABTR2, SAR405838-treated) were treated with ABT-263 or vehicle control for 24 h for apoptosis analysis by flow cytometry with Annexin V/P.I. double staining. Data (mean ± SD) are from triplicates, including both early (Annexin V-positive/PI-negative) and late (Annexin V-positive/PI-positive) apoptotic cells. D, Immunoblotting of Bcl-2 family proteins for in vitro and in vivo RS4;11 resistant sublines and control lines. GAPDH was used as the loading control.