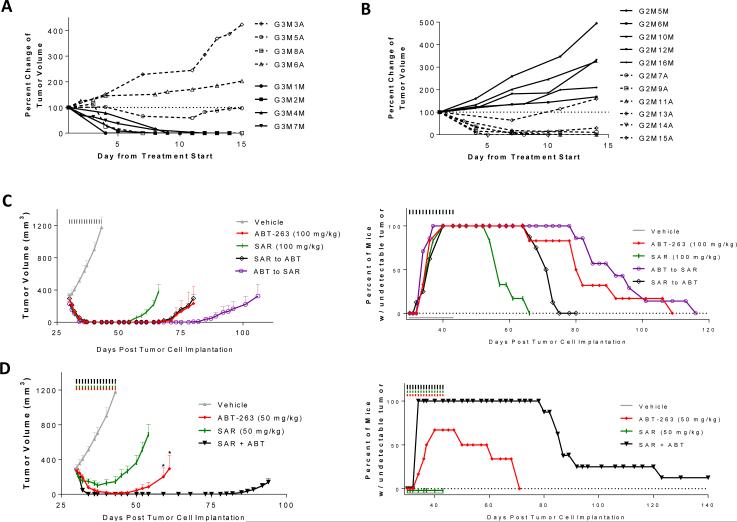
Figure 6.
Antitumor activity of ABT-263 and SAR405838 alone, sequential treatment of the two drugs and their combination in the RS4;11 xenograft model. A, Antitumor activity of ABT-263 and SAR405838 in regrown tumors initially treated with ABT-263. When regressed tumors initially treated with ABT-263 daily for 21 days at 100 mg/kg via oral gavage (SI Fig. 3A) regrew to 100-200 mm3, they were retreated with either SAR405838 at 100 mg/kg (solid lines) or ABT-263 (dashed lines) at 150 mg/kg, both via oral gavage, daily for 14 days. Data are presented as percent change of the tumor volume for each mouse. B, Antitumor activity of ABT-263 and SAR405838 in regrown tumors initially treated with SAR405838. When regressed tumors initially treated with SAR405838 for 21 days at 100 mg/kg (SI Fig. 3D) regrew to 100-200 mm3, they were retreated with either ABT-263 at 100 mg/kg (dashed lines) or SAR405838 (solid lines) at 200 mg/kg for 14 days. Data are presented as percent change of the tumor volume for each mouse. C, Antitumor activity of single agents and sequential treatment. Mice were treated via oral gavage with vehicle, ABT-263 or SAR405838 for 14 days, or with one drug for 7 days, immediately followed with the other drug for an additional 7 days. Percentage of tumor-free survival of mice in the RS4;11 efficacy experiment shown on the left. D, Antitumor activity of ABT-263, SAR405838 and their combination. When tumors grew to approximately 300 mm3, mice were treated with vehicle, ABT-263, SAR405838 or their combination at 50 mg/kg for 14 days via oral gavage (*, P <0.05; t test for comparison of ABT-263 single agent treatment at 50 mg/kg to the combination treatment). Percentage of tumor-free survival of mice in the RS4;11 efficacy experiment shown on the left.