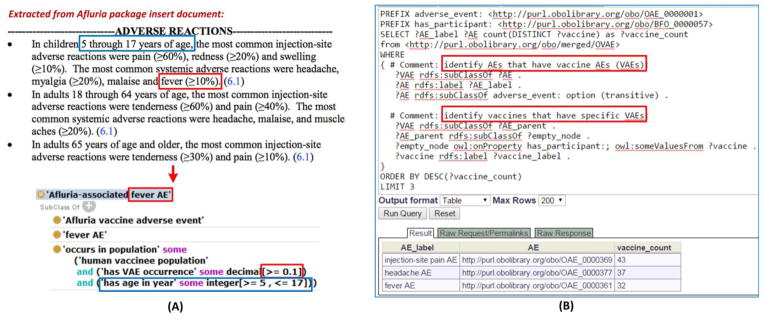
Figure 4. OVAE representation and query of AEs associated with FDA licensed vaccines.
(A) OVAE representing Afluria VAEs reported in FDA vaccine package insert. The top screen shows the adverse reactions of Afluria as recorded in the FDA vaccine package insert document of Afluria. The bottom screen (extracted from a view using the Protégé OWL editor) shows how OVAE represents the >=10% occurrence of ‘Afluria-associated fever AE’ in children 5 through 17 years of age. The OVAE representation matches the information in the FDA vaccine package insert document. (B) SPARQL query of OVAE for the top 3 AEs associated with the highest numbers of vaccines. As shown in (A), ‘Afluria-associated fever AE’ is a cross product of ‘Afluria vaccine adverse event’ and ‘fever AE’, which provides the query strategy for identifying the vaccine (Afluria) and the specific AE (‘fever AE’). The query was performed using the Ontobee SPARQL web program: http://www.ontobee.org/sparql.