Figure 7. Stabilization of p27 by Cx50 during the Cell Cycle and Reduction of p27/p57 in Cx50-Deficient Lens.
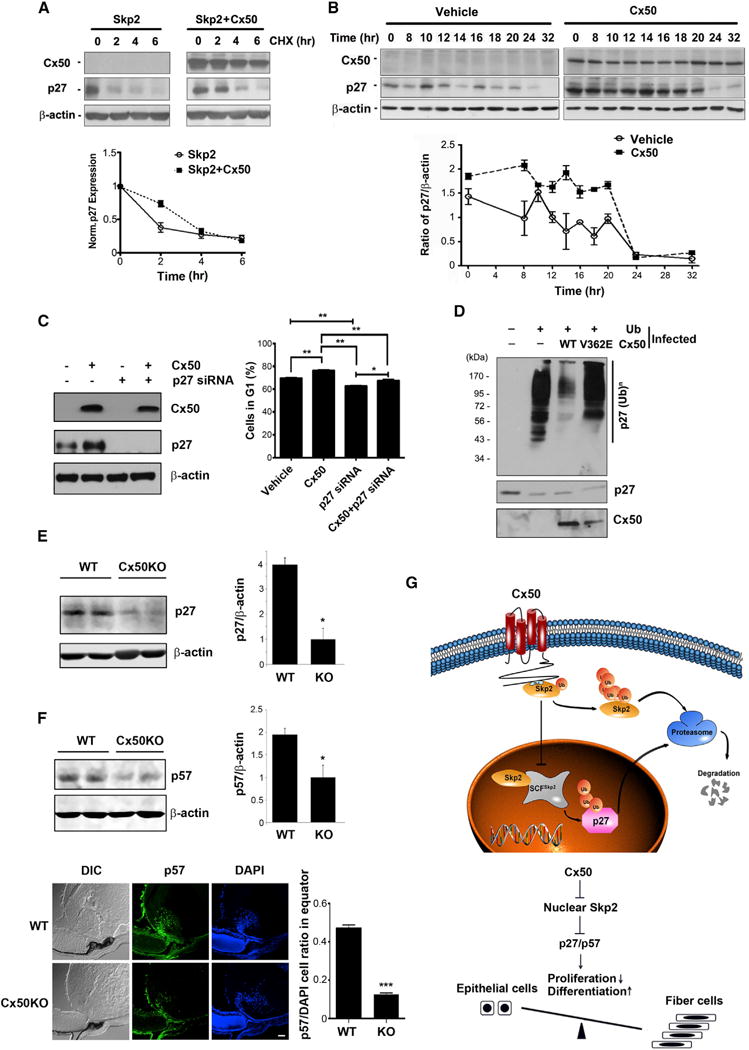
(A) Cx50 stabilizes p27 at G0/G1 phase. The protein levels were normalized to 0 hr.
(B) Cx50 increases the expression level of p27 during cell-cycle progression. The expression level of protein was normalized to 0 hr of RCAS(A) vehicle control.
(C) Knocking down p27 promotes the cell cycle that is arrested by Cx50. The cell population was determined by FACS analysis with PI staining. (D) Cx50 decreases ubiquitination level of p27. The protein levels were examined by western blotting (lower).
(E) Decreased levels of p27 in Cx50-knockout lens. Lens lysates from 3-day-old WT and Cx50 knockout mice were immunoblotted (left) and quantified (right).
(F) Decreased levels of p57 in the bow region of Cx50-knockout lens. Lens lysates of 3-day old WT and Cx50 KO mice were immunoblotted with anti-p57 or β-actin antibody (upper left) and quantified (upper right). The frozen tissue sections of 3-day-old lenses from WT or Cx50 knockout mice were immunofluo-rescence-labeled (lower left) and quantified (lower right). Scale bar represents 50 μm.
(G) Schematic model illustrates the interaction and mechanistic roles of Cx50 and Skp2 in regulating lens cell differentiation. Left: Cx50 interacts with Skp2 in the cytosol, leading to cytoplasmic retention of Skp2, thereby preventing it from translocating into nucleus. Reduced nuclear Skp2 leads to decreased ubiquitination of p27/p57 in nucleus and less degradation of p27/p57. Right: the increased p27 due to the retention of Skp2 in cytosol by Cx50 leads to cell-cycle arrest and promotion of lens cell differentiation.
