Abstract
Background
Hereditary angioedema with normal C1 inhibitor levels (HAE-N) is associated with a Factor XII mutation in 30% of subjects; however, the role of this mutation in the pathogenesis of angioedema is unclear.
Objective
We sought evidence of abnormalities in the pathways of bradykinin formation and bradykinin degradation in the plasma of patients with HAE-N both with and without the mutation.
Methods
Bradykinin was added to plasma, and its rate of degradation was measured by using ELISA. Plasma autoactivation was assessed by using a chromogenic assay of kallikrein formation. Plasminogen activator inhibitors (PAIs) 1 and 2 were also measured by means of ELISA.
Results
PAI-1 levels varied from 0.1 to 4.5 ng/mL (mean, 2.4 ng/mL) in 23 control subjects, from 0.0 to 2 ng/mL (mean, 0.54 ng/mL) in patients with HAE-N with a Factor XII mutation (12 samples), and from 0.0 to 3.7 ng/mL (mean, 1.03 ng/mL) in patients with HAE-N without a Factor XII mutation (11 samples). PAI-2 levels varied from 25 to 87 ng/mL (mean, 53.8 ng/mL) in control subjects and were 0 to 25 ng/mL (mean, 4.3 ng/mL) in patients with HAE-N with or without the Factor XII mutation. Autoactivation at a 1:2 dilution was abnormally high in 8 of 17 patients with HAE-N (4 in each subcategory) and could be corrected by supplemental C1 inhibitor in 4 of them. Bradykinin degradation was markedly abnormal in 1 of 23 patients with HAE-N and normal in the remaining 22 patients.
Conclusions
Bradykinin degradation was normal in all but 1 of 23 patients with HAE-N studied. By contrast, there was a marked abnormality in PAI-2 levels in patients with HAE-N that is not seen in patients with C1 inhibitor deficiency. PAI-1 levels varied considerably, but a statistically significant difference was not seen. A link between excessive fibrinolysis and bradykinin generation that is estrogen dependent is suggested.
Keywords: Hereditary angioedema, C1 inhibitor, bradykinin, plasminogen activator inhibitor
Hereditary angioedema with normal C1 inhibitor levels (HAE-N; previously known as type III HAE)1,2 is associated with a mutation in Factor XII in approximately 30% of patients and is strikingly estrogen dependent.3,4 However, the relationship of this mutation to the pathogenesis of angioedema is uncertain; initial evidence to suggest that the mutated Factor XII is abnormally active5 was refuted soon thereafter.6 What is currently known is that the likely mediator of the swelling is bradykinin based on (1) the striking similarity of the clinical presentation to that of type I and II hereditary angioedema (HAE)7 and (2) successful control of acute symptoms by blocking the bradykinin receptor8 or inhibiting plasma kallikrein.9 Curiously, infusion of C1 inhibitor (C1-INH) can also be effective acutely, suggesting that supranormal C1-INH levels can be therapeutic.4
We have considered the possibility that the underlying abnormality of this form of HAE is a deficiency of some other plasma inhibitor, the consequence of which might be enhanced activation of the plasma bradykinin-forming cascade when compared with normal values. Here we report that the main abnormality in patients with HAE-N is a deficiency of plasminogen activator inhibitor (PAI) 2 with diminished PAI-1 levels while all other parameters of activation or inhibition of the plasma bradykinin-forming cascade are normal. There was no distinction based on the presence or absence of the Factor XII mutation.
METHODS
Patients and sample collection
The diagnosis of HAE was confirmed by a clinical presentation of recurrent angioedema in the absence of urticaria, plus low C1-INH and/or function levels10 by using a commercial assay performed at the site of origin of the samples. Patients with HAE-N had a clear family history of HAE, normal C1-INH protein and function levels, and in most instances striking estrogen dependence. Citrated plasma from 23 patients with HAE-N, 23 patients with type I HAE, and 23 healthy control subjects was separated by means of centrifugation of freshly collected blood at 2000 rpm for 10 minutes at 4°C. All samples were immediately placed in aliquots and stored at −80°C. Samples were handled similarly at all participating sites and shipped overnight on dry ice. The protocol was approved by the ethics committees and data protection agencies at all participating sites.
ELISA to measure C1-INH protein levels in plasma
Immulon 2HB plates (Thermo Scientific, Waltham, Mass) were coated with 5 μg/mL polyclonal antibody to C1-INH. After blocking with 1% BSA in PBS, samples and standards were added and incubated at room temperature for 1 hour. Bound C1-INH was probed with alkaline phosphatase–conjugated mAb to C1-INH, followed by color development with 5-bromo-4-chloroindolyl phosphate/nitroblue tetrazolium (BCIP/NBT).
Functional assay for C1-INH based on inhibition of kallikrein and Factor XIIa
Immulon 2HB plates were coated with 5 μg/mL avidin in coating buffer (100 μL) overnight at 4°C. Plates were washed 3 times with PBS-Tween (200 μL of each). Subsequently, 200 μL of 1% BSA in PBS was added to block the unused sites. The plates were incubated at 37°C for 1 hour and washed 3 times with PBS-Tween (200 μL of each). Samples or standards were added to the plates along with biotinylated protein (25 μL of standards or samples, 25 μL of biotinylated Factor XII or biotinylated kallikrein [1 μg/mL], and 50 μL of binding buffer), mixed, and incubated at 37°C for 1 hour. After incubation, plates were washed 3 times with PBS-Tween (200 μL of each). A polyclonal antibody to C1-INH was added, followed by incubation at room temperature for 1 hour. The wells were washed with PBS-Tween 3 times. Alkaline phosphatase–conjugated secondary antibody was added, and the samples were incubated at room temperature for 1 hour, followed by color development with the phosphatase substrate BCIP/NBT. The OD at 450 nm was read, and calculations were performed by using the standard curve.11
Functional assay for α2-macroglobulin based on kallikrein inhibition
The functional assay for α2-macroglobulin was developed in the same way as for C1-INH by using biotinylated kallikrein. After incubation, antibodies to α2-macroglobulin were used for detection, followed by color development, as described above.
Bradykinin degradation in plasma
For bradykinin degradation studies, samples were prepared by incubating a known amount of bradykinin (300 ng) with each plasma (patients with HAE-N and healthy control subjects), and aliquots were collected at the indicated time. The proteins in the aliquots were precipitated with ice-cold ethanol and centrifuged for 1 hour at 10,000 rpm in a microcentrifuge at 4°C, and the supernatant containing free bradykinin was collected. The supernatant was then evaporated with a centrifugal concentrator to dryness and resuspended in EIA buffer. Bradykinin enzyme immunoassay was performed with an assay kit from Peninsula Laboratories (San Carlos, Calif), according to the manufacturer’s recommendations. The concentrations of bradykinin present in samples were calculated by using a standard curve and are expressed as picograms per milliliter.
Prekallikrein activation assay
Activation of prekallikrein to form kallikrein on incubation of plasma in room temperature without any added activators was considered to represent “spontaneous” activation.12 Kallikrein activity assays were performed, as described previously.13
Measurement of protein levels of plasma inhibitors
Protein levels of β2-glycoprotein I, α2-antiplasmin, activated protein C inhibitor, and inter-α-trypsin inhibitor in patients’ samples were compared with control values by means of Western blotting, ELISA, or both.
SDS-PAGE and Western blotting
SDS-PAGE was performed with the buffer system of Laemmli.14 Gradient gels (4–20%) were used for separation of proteins. After electrophoresis, the proteins were transferred to nitrocellulose membranes overnight. The membranes were then incubated with blocking buffer (1% BSA in PBS) for 1 hour after blocking and probed with mAbs for an additional 1 hour. Bound probes were visualized by incubating the membranes with alkaline phosphatase–conjugated secondary antibodies, followed by color development in BCIP/NBT.
ELISA to measure PAI-1 and PAI-2 levels in plasma
ELISA kits for the measurement of PAI-1 and PAI-2 levels were obtained from Cloud-Clone (Houston, Tex). The assays were initially performed, as suggested by the manufacturer, with a plasma dilution of 1:100. However, the results from 1:100 and 1:50 dilutions of plasma from patients with HAE-N were all less than the level of detection. Therefore we repeated the assays by using plasma dilutions of 1:10 and 1:5, and the values obtained were used to calculate nanograms per milliliter. Values from normal plasma and plasma from patients with type I/II HAE exceeded the standard curve at dilutions of less than 1:50.
Statistical analysis
The primary outcomes of interest were PAI-1 and PAI-2 levels among the 4 groups. Associations between PAI-1 and PAI-2 levels by disease group were evaluated by using linear regression models. Model assumptions were checked graphically. Pairwise comparisons between groups were evaluated by using contrast statements with a Tukey adjustment to control for multiple comparisons. All analyses were conducted in SAS software (version 9.3; SAS Institute, Cary, NC).
RESULTS
Quantitation of functional C1-INH
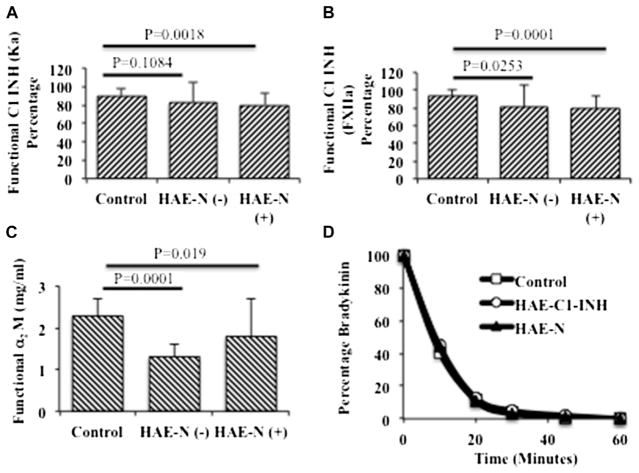
We first considered the possibility that patients’ C1-INH levels could be normal based on inhibition of activated C1 yet abnormal if assayed based on inhibition of kinin-forming enzymes. The results for 17 patients who were assayed and compared with 17 healthy control subjects are shown in Fig 1, A and B. Some patients with HAE-N had inhibitory levels of 60% to 75%, and this was evident whether we assayed inhibition of kallikrein or activated Factor XII. This might represent secondary depletion of C1-INH in some subjects. Although functional C1-INH levels of 40% of normal or less are typical of symptomatic patients with type I or II HAE, C1-INH levels determined by means of inhibition of Factor XIIa or kallikrein were significantly decreased in patients with HAE-N irrespective of whether the Factor XII mutation was present or absent.
FIG 1.
Assays to determine functional C1-INH levels, functional α2-macroglobulin levels, and degradation of bradykinin in plasma of patients with HAE-N and control subjects. A, C1-INH functional assay based on inhibition of kallikrein. B, C1-INH functional assay based on inhibition of Factor XIIa. C, α2-Macroglobulin functional assay based on inhibition of kallikrein. D, Degradation of bradykinin by plasma kininases. Assays were performed as described in the Methods section. HAE-N (+) indicates a Factor XII mutation is present, and HAE-N (−) indicates there is no Factor XII mutation. FXIIa, Factor XIIa.
Assay of other possible inhibitors of kinin-forming enzymes
We next developed a functional assay for α2-macroglobulin, as described in the Methods section; applied it to plasma of patients with HAE-N; and compared it with plasma of healthy control subjects. The results are shown in Fig 1, C. Similar to C1-INH, functional α2-macroglobulin in patients’ plasma seemed diminished compared with that in normal control plasma and reached statistical significance. However, all exceeded 60% of normal, so that a deficiency that might explain the occurrence of angioedema in these patients seemed unlikely. There are additional plasma inhibitors, some of which are reported to inhibit bradykinin formation. The ones we assayed are listed in Table I, including a reference regarding functional activity that would be relevant.15–18 Results for all of these, including activated protein C inhibitor, β2-glycoprotein I, α2-antiplasmin, and inter-α-trypsin inhibitor, were normal (data not shown).
TABLE I.
Inhibitors that were found to be normal in patients with HAE-N
| Inhibitors | Normal protein assay | Relevant inhibitory activity |
|---|---|---|
| α2-Macroglobulin* | + | Kallikrein14 |
| α2-Antiplasmin | + | Plasmin15 |
| β2-Glycoprotein 1 | + | Factor XIIa16 |
| Protein Ca inhibitor | + | Kallikrein17 |
| Inter-α-trypsin inhibitor | + | None known |
The functional assay (means) was 60% and 74% of normal for patients without and with HAE-N, respectively.
Bradykinin degradation
Because we were unable to find a major inhibitor deficiency in plasma of patients with HAE-N, we considered the possibility that increased bradykinin levels could result from a mutation in a key kininase analogous to that seen in patients with angioedema caused by ingestion of angiotensin-converting enzyme inhibitors.19 The rate of bradykinin degradation was determined in plasma of 23 patients with HAE-N, and 22 were indistinguishable from normal, as shown in Fig 1, D. There was one exception in a patient without a Factor XII mutation in whom the rate of bradykinin degradation was markedly reduced and will require assessment of individual kininases to identify a putative abnormality. On the other hand, in general, we concluded that patients with HAE-N do not have abnormal bradykinin degradation and that if increased bradykinin levels are responsible for angioedema, it must be due to overproduction.
Assessment of plasma instability in patients with HAE-N compared with those with type I/II HAE
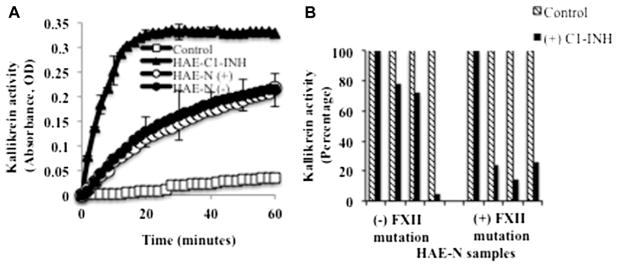
Plasma of patients with type I or II HAE is intrinsically unstable, such that on prolonged incubation at 37°C, kallikrein activity evolves with concomitant bradykinin formation. This occurs in the absence of any initiating surface, is independent of Factor XII,12 and appears to be due to autoactivation of the prekallikrein high-molecular-weight kininogen complex in a phosphate milieu when C1-INH levels are inadequate to maintain control.12 When we assayed undiluted plasma of patients with HAE-N in the same fashion, it was normal. Because normal plasma will resemble that of plasma from patients with type I/II HAE if it is diluted 1:3 or more, we compared 1:2 dilutions of plasma from patients with HAE-N with that of healthy control subjects. Gradual evolution of kallikrein activity was seen in plasma from 8 of 17 patients with HAE-N but did not occur in any of the healthy control subjects (Fig 2, A). It should be noted that a 1:3 dilution of normal plasma (C1-INH at 33% of normal values) demonstrates instability. Of the 8 abnormal plasma samples from patients with HAE-N, 4 reverted to normal if supplemented with 100 μg/mL C1-INH protein (Fig 2, B), and 4 did not. Thus a subtle abnormality of bradykinin formation was observed in patients with HAE-N, far less than that seen when type I or II HAE plasma was tested, and this was not seen in every patient sample (see Table E1 in this article’s Online Repository at www.jacionline.org).
FIG 2.
Spontaneous activation of the bradykinin-forming pathway in patients with HAE-N compared with patients with HAE type I and control subjects (A) and inhibition of activation by using C1-INH (B). Plasma was diluted 1:2 with HEPES-buffered saline and incubated at room temperature, and kallikrein activity was measured by using a chromogenic substrate (S2302; diaPharma, West Chester Township, Ohio). About half of the samples from patients with HAE-N were activated at 1:2 dilution, and the curves looked similar. Thus a representative profile is shown. For inhibition studies (Fig 2, B), selected plasma samples with spontaneous activation (8 samples, 4 from each group) were incubated in the presence of 100 μg/mL purified C1-INH, and kallikrein activity was measured after 1 hour. FXII, Factor II.
Abnormalities of PAI levels
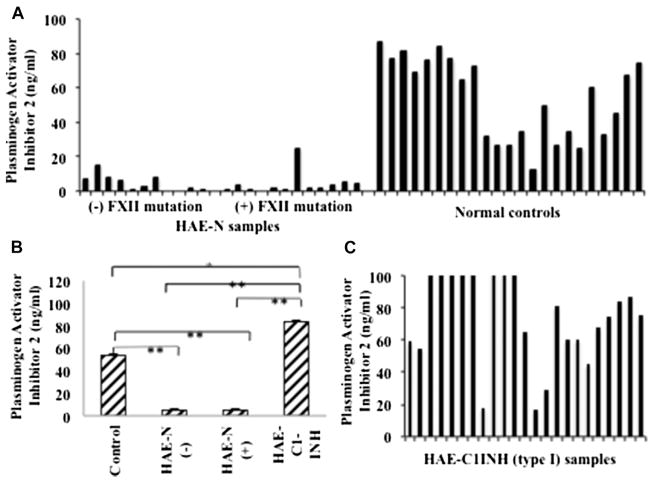
Because the plasma demonstrated no striking abnormalities within the “intrinsic” pathway for activation of the bradykinin-forming cascade, we turned to possible exogenous activators. Possibilities include release of heat shock protein 90 from endothelial cells to activate the prekallikrein-high molecular weight kininnogen (HK) complex20 and secondarily activate Factor XII by the kallikrein thus formed21,22 or activation of the fibrinolytic pathway (ie, formation of plasmin within the plasma through release of tissue plasminogen activator or urokinase from sources such as endothelial cells).23 The latter considerations led us to examine inhibitors of plasminogen activators in plasma that are not known to act on kallikrein or the various forms of activated Factor XII. Results for PAI-2 are shown in Fig 3. Twenty-three patients with HAE-N were tested, and a striking deficiency of PAI-2 is noted when compared with levels in 23 healthy control subjects or 23 patients with type I/II HAE. The insert is a bar graph showing the mean and SE for each. The P value compared with normal is less than .001. There was not a significant difference in PAI-2 levels for the 2 HAE-N groups (P =1.00). Samples from control subjects had significantly higher PAI-2 levels relative to those from patients with HAE-N with Factor XII mutations or those from patients with HAE-N with no Factor XII mutations (P < .001 for both). Similarly, samples from patients with type I/II HAE had significantly higher PAI-2 levels relative to those from patients with HAE-N with or without the Factor XII mutation (P <.001 for both). However, control samples had significantly lower PAI-2 levels relative to those of patients with type I/II HAE (P =.003). Our data concerning PAI-2 levels suggest a profound and perhaps underlying deficiency.
FIG 3.
PAI-2 levels in plasma of patients with HAE-N and control subjects. PAI-2 levels in plasma of patients and control subjects were measured by means of ELISA. A, Each bar represents individual subjects (HAE-N) expressed as nanograms per milliliter. B, Means ± SEs of 23 control samples, 11 samples from patients with HAE-N without the Factor XII mutation and 12 samples from patients with HAE-N with the Factor XII mutation, and 23 samples from patients with HAE type I. C, Individual values for patients with HAE type I. Values that exceeded the detection limit of 100 are shown as 100. *Significant difference at P < .01 and **significant difference at P < .001. FXII, Factor II.
The assay procedures for PAI-1 and PAI-2 recommend a plasma dilution of 1:100, and at that dilution, all HAE-N samples had values of less than the level of detection, whereas normal plasma or plasma of patients with type I/II HAE was readily quantitated. We then repeated the assay for HAE-N samples using the same dilutions of 1:10 and 1:5, and the results were used to calculate the values expressed as nanograms per milliliter.
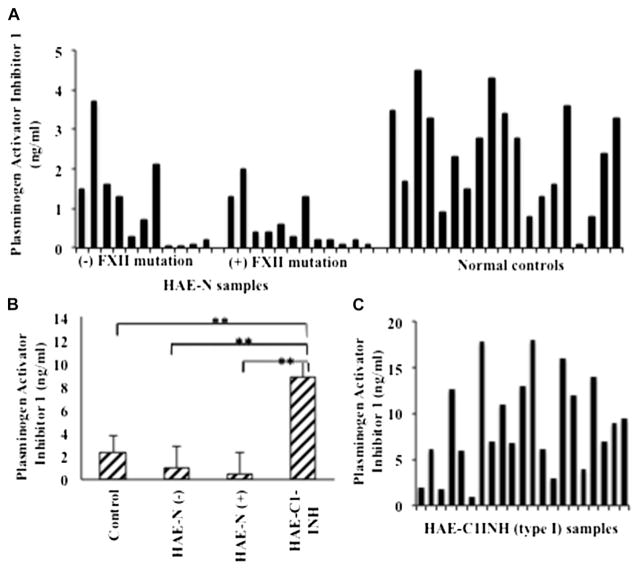
We then tested PAI-1, levels of which were previously found to be low in patients with type I and II HAE.24 An autosomal recessive deficiency of this protein has been described with bleeding rather than angioedema as a manifestation.25,26 Our results, as shown in Fig 4, indicate that there were no significant differences in PAI-1 levels between samples from healthy control subjects and those from patients with HAE-N with or without the Factor XII mutation (P =.680 and .404, respectively) taken as a group, although there was considerable variability within patients with HAE-N. The difference in PAI-1 levels also did not differ significantly between the 2 HAE-N subgroups (P = .983). However, samples from patients with type I/II HAE had significantly higher PAI-1 levels relative to those from healthy control subjects, patients with HAE-N with the Factor XII mutation, and patients with HAE-N without the Factor XII mutation (P < .001 for all comparisons).
FIG 4.
PAI-1 levels in plasma of patients with HAE-N and control subjects. PAI-1 levels in plasma of patients and control subjects were measured by means of ELISA. A, Each bar represents individual subjects (HAE-N) expressed as nanograms per milliliter. B, Means ± SEs of 19 control samples, 11 samples from patients with HAE-N without the Factor XII mutation and 12 samples from patients with HAE-N with the Factor XII mutation, and 20 samples from patients with HAE type I. C, Individual values for patients with HAE type I. **Significant difference at P < .001. FXII, Factor II.
DISCUSSION
HAE-N was first described 14 years ago1,2 and is associated with a mutation in Factor XII within the proline-rich region of the molecule, the contribution of which to function, if any, is unknown.6 Most common is a Lys substituted for Thr within exon 9,3 but an Arg substituted for Thr3 or deletions or duplications within the same region of the molecule have been reported.27–29 Although these mutants are clearly a marker of the disease (no such mutation is seen in any other entity), its relationship to the pathogenesis of HAE-N is unclear. There is some preliminary evidence to indicate that the mutated forms are more susceptible to plasmin cleavage and perhaps to activation (Coen Maas, personal communication), yet patients lacking the mutation are not distinguishable from those with it. However, bradykinin is considered a likely candidate as a cause of the angioedema because of the similarity in presentation to type I/II HAE and responsiveness acutely to some of the same medications.
If the focus is on Factor XII, activation is dependent on autoactivation when bound to initiating surfaces that are typically negatively charged,30 followed by feedback activation by the kallikrein produced31; the latter is dominant kinetically.32 By using dextran sulfate (10–20 μg/mL) as an initiator, an initial publication suggested increased activity of the mutated Factor XII5; however, this was refuted soon thereafter,6 and we found no evidence of any abnormality of bradykinin production through contact activation of patients’ plasma with dextran sulfate (data not shown) as long as the C1-INH level was more than 80% of normal. A more recent publication focusing only on HAE-N with mutated factor XII reports increased autoactivatability rather than enhanced activity of the factor XIIa33 if the surface (dextran sulfate) is at a low level (less than 10 μg/mL) due to absent glycosylation of the same amino acid that yields a new plasminogen cleavage site. We compared the intrinsic lability of HAE-N with that of types I and II HAE. It is far more stable and more closely approaches normal plasma, although some patients demonstrate lability at a 1:2 dilution (Fig 2). This might relate in part to the fact that C1-INH levels are actually diminished in some patients to between 60% to 80% of normal values (Fig 1, A and B), and α2-macroglobulin levels are low as well (Fig 1, C). Yet it seems legitimate to consider this HAE with “normal” C1-INH levels because such levels are not low enough to cause symptoms (C1-INH levels >40% are unlikely to lead to symptoms), the C4 level is typically normal, and the entity is clearly distinguishable from type I and II HAE, even if the Factor XII mutation is absent. However, the latter cannot be diagnosed unless the family history is clear.34
We have demonstrated that there is no kininase abnormality in 22 of 23 patients, but there is one exception that requires further assessment. We assayed other possible inhibitor deficiencies in all samples from patients with HAE-N and found (or confirmed) normal α2-macroglobulin, α2-antiplasmin, protein Ca inhibitor, β2-glycoprotein I, and inter-α-trypsin inhibitor levels. α2-Macroglobulin was assayed based on both protein levels and functional assessment, with the latter 4 assayed based on protein level only.
The results from our studies on the fibrinolytic cascade showed one striking difference between patients with HAE-N and healthy control subjects. Plasma levels of PAI-2 in patients with HAE-N were just as low as levels of C1-INH in patients with type I and II HAE, suggesting that it could be a deficient or depleted protein in those with HAE-N. In fact, the values reported for healthy control subjects and patients with type I/II were calculated from the results obtained by using a dilution of 1:100 and 1:50, although samples from patients with HAE-N were assayed by using dilutions of 1:10 and 1:5. Previous reports have suggested that PAI-2 levels were either too low to measure in plasma of healthy subjects but were increased during pregnancy35 or that normal values ranged from less than the level of detection to as high as 36 ng/mL.36 With current methodology, the normal values we obtained ranged from 15 to 80 ng/mL; 13 of 23 patients with type I/II HAE had levels within the normal range, and 10 of 23 had levels that exceeded it.
It is also of interest that those with and without the Factor XII mutation have equally abnormal results. Search for a gene mutation within the coding region and/or transcription regulators of PAI-2 is warranted, particularly in those lacking the factor XII mutation. Nevertheless, similar samples we recently received from Brazilian families in whom most were treated with tranexamic acid, an antifibrinolytic, or progestin had PAI-2 levels close to normal, thus depletion rather than a synthetic abnormality is likely which will be reported separately. Although some patients have very low PAI-1 levels as well, some have level close to normal, and we speculate that if the fibrinolytic cascade is partially active in these patients, PAI-1 levels might be depleted because of the low levels of PAI-2. As a group, mean PAI-1 levels in patients with HAE-N were not statistically different from normal levels. It will be of interest to compare levels in individual patients during attacks of angioedema because all samples assayed by us were examined at a time the disease was quiescent. Plasmin α2-antiplasmin complex levels would also be of particular interest.
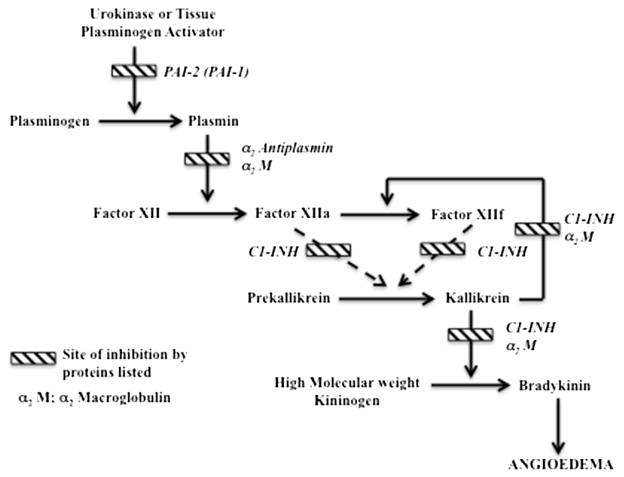
In summary, multiple minor abnormalities in the pathways for bradykinin generation were noted in plasma samples of patients with HAE-N, and bradykinin degradation was normal, except for one exceptional case. On the other hand, there were low PAI levels, particularly PAI-2, which were close to zero in most patients, and might represent a key abnormality. A link to the bradykinin-forming cascade might be through plasmin activation of Factor XII,37 and those subjects with the Factor XII mutation could have facilitated activation through this route (Fig 5). It should be noted that the intrinsic fibrinolytic pathway emanating from Factor XIIa is very weak38,39 and cannot account for the aforementioned inferences. It would require an exogenous source of tissue plasminogen activator, urokinase, or both to convert plasminogen to plasmin stimulated perhaps by cytokines, estrogen, or bradykinin itself.40,41
FIG 5.
Proposed mechanism of angioedema formation in patients with HAE-N. Assuming a source of urokinase and/or tissue plasminogen activator (eg, endothelial cell activation), deficiency of PAI-2 and diminished levels of PAI-1 (in some cases, such as Fig 4, A) can lead to excessive plasmin formation. The connection to the bradykinin-forming cascade is through plasmin activation of Factor XII.37 In those with the Factor XII mutation, susceptibility to plasmin activation can be enhanced. Later steps include conversion of prekallikrein to kallikrein by Factor XIIa or Factor XIIf,37 the kallikrein feedback activation of Factor XII,21,31 and kallikrein cleavage of HK to release bradykinin.
Supplementary Material
Clinical implications.
Deficiency of PAI-2 in patients with HAE with normal C1-INH levels suggests an initiation mechanism for angioedema formation in which plasmin activates Factor XII, leading to bradykinin production.
Acknowledgments
Supported by CSL Behring.
Abbreviations used
- BCIP/NBT
5-Bromo-4-chloroindolyl phosphate/nitroblue tetrazolium
- C1-INH
C1 inhibitor
- HAE
Hereditary angioedema
- HAE-N
Hereditary angioedema with normal C1 inhibitor levels
- HK
High molecular weight kininnogen
- PAI
Plasminogen activator inhibitor
Footnotes
Disclosure of potential conflict of interest: K. Joseph has received research support from CSL Behring, Shire HGT, and Dyax. B. G. Tholanikunnel has received research support from CSL Behring. B. Wolf has received research support from the National Institutes of Health/NCRR (grant no. UL1RR029882). K. Bork has received research support from CSL Behring and Shire HGT. A. P. Kaplan has received research support from CSL Behring, Shire HGT, and Dyax and has received lecture fees from Shire HGT, and Dyax.
References
- 1.Bork K, Barnsterdt SE, Koch P, Traupe H. Hereditary angioedema with normal C1 inhibitor activity in women. Lancet. 2000;356:213–7. doi: 10.1016/S0140-6736(00)02483-1. [DOI] [PubMed] [Google Scholar]
- 2.Binkley KE, Davis A., 3rd Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol. 2000;106:546–50. doi: 10.1067/mai.2000.108106. [DOI] [PubMed] [Google Scholar]
- 3.Dewald G, Bork K. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun. 2006;343:1286–9. doi: 10.1016/j.bbrc.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 4.Bork K, Wulff K, Hardt J, Witzke G, Staubach P. Hereditary angioedema caused by missense mutations in the factor XII gene: clinical features, trigger factors, and therapy. J Allergy Clin Immunol. 2009;124:129–34. doi: 10.1016/j.jaci.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 5.Cichon S, Martin L, Hennies HC, Müller F, Van Driessche K, Karpushova A, et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 2006;79:1098–104. doi: 10.1086/509899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bork K, Kleist R, Hardt J, Witzke G. Kallikrein-kinin system and fibrinolysis in hereditary angioedema due to factor XII gene mutation Thr 309 lys. Blood Coagul Fibrinolysis. 2009;20:325–32. doi: 10.1097/MBC.0b013e32832811f8. [DOI] [PubMed] [Google Scholar]
- 7.Bork K, Gul D, Hardt J, Dewald G. Hereditary angioedema with normal C1 inhibitor: clinical symptoms and course. Am J Med. 2007;120:987–92. doi: 10.1016/j.amjmed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Bouillet L, Boccon-Gibod I, Ponard D, Drouet C, Cesbron JY, Dumestre-Perard C, et al. Bradykinin receptor 2 antagonist (icatibant) for hereditary angioedema type III attacks [letter] Ann Allergy Asthma Immunol. 2009;103:448. doi: 10.1016/S1081-1206(10)60369-9. [DOI] [PubMed] [Google Scholar]
- 9.Cronin JA, Maples KM. Treatment of an attack of type III hereditary angioedema with ecallantide [letter] Ann Allergy Asthma Immunol. 2012;108:61–2. doi: 10.1016/j.anai.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008;359:1027–36. doi: 10.1056/NEJMcp0803977. [DOI] [PubMed] [Google Scholar]
- 11.Joseph K, Bains S, Tholanikunnel BG, Bygum A, Aabom A, Koch C, et al. A novel assay to diagnose hereditary angioedema utilizing inhibition of bradykinin-forming enzymes. Allergy. 2015;70:115–9. doi: 10.1111/all.12520. [DOI] [PubMed] [Google Scholar]
- 12.Joseph K, Tholanikunnel BG, Bygum A, Ghebrehiwet B, Kaplan AP. Factor XII independent activation of the bradykinin-forming cascade: Implications for the pathogenesis of hereditary angioedema types I and II. J Allergy Clin Immunol. 2013;132:470–5. doi: 10.1016/j.jaci.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Joseph K, Tholanikunnel T, Kaplan AP. Treatment of episodes of hereditary angioedema with C1 inhibitor: serial assessment of observed abnormalities of the plasma bradykinin-forming pathway and fibrinolysis. Ann Allergy Asthma Immunol. 2010;104:50–4. doi: 10.1016/j.anai.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Harpel PC, Lewin MF, Kaplan AP. Distribution of plasma kallikrein between C1-inhibitor and α2-macroglobulin in plasma utilizing a new assay for α2-macroglobulin-kallikrein complexes. J Biol Chem. 1985;260:4257–63. [PubMed] [Google Scholar]
- 16.Collen D. Identification and some properties of a new fast-reacting plasmin inhibitor in human plasma. Eur J Biochem. 1976;69:209–16. doi: 10.1111/j.1432-1033.1976.tb10875.x. [DOI] [PubMed] [Google Scholar]
- 17.Schousboe I. β2-Glycoprotein I: a plasma inhibitor of the contact activation of the intrinsic blood coagulation pathway. Blood. 1985;66:1086–91. [PubMed] [Google Scholar]
- 18.Espana F, Berrettini M, Griffin JH. Purification and characterization of plasma protein C inhibitor. Thromb Res. 1989;55:369–84. doi: 10.1016/0049-3848(89)90069-8. [DOI] [PubMed] [Google Scholar]
- 19.Malde B, Regalado J, Greenberger PA. Investigation of angioedema associated with the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Ann Allergy Asthma Immunol. 2007;98:57–63. doi: 10.1016/S1081-1206(10)60860-5. [DOI] [PubMed] [Google Scholar]
- 20.Joseph K, Tholanikunnel BG, Kaplan AP. Heat shock protein 90 catalyzes activation of the prekallikrein-kininogen complex in the absence of Factor XII. Proc Natl Acad Sci U S A. 2002;99:896–900. doi: 10.1073/pnas.022626899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochrane CG, Revak SD, Wuepper KD. Activation of Hageman factor in solid and fluid phases: a critical role of kallikrein. J Exp Med. 1973;138:1564–83. doi: 10.1084/jem.138.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn JT, Silverberg M, Kaplan AP. The cleavage and formation of activated Hageman factor by autodigestion and by kallikrein. J Biol Chem. 1982;257:1779–84. [PubMed] [Google Scholar]
- 23.Levin EG, Loskutoff DJ. Cultured bovine endothelial cells produce both urokinase and tissue-type plasminogen activators. J Cell Biol. 1982;94:631–6. doi: 10.1083/jcb.94.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bork K, Braddin K, Heinburger N, Lindhoff E, Mosch G, Witzke G. Reduced PAI-1 activities in patients with angioedema (AE) due to C1 inhibitor deficiency [abstract] Thromb Haemost. 1991;65:1271. [Google Scholar]
- 25.Van Geffen M, Cugno M, Lap P, Loof A, Cicardi M, van Heerde W. Alterations of coagulation and fibrinolysis in patients with angioedema due to C1-inhibitor deficiency. Clin Exp Immunol. 2011;167:472–8. doi: 10.1111/j.1365-2249.2011.04541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta R, Shapiro AD. Plasminogen activator inhibitor type I deficiency. Hemophilia. 2008;14:1255–60. doi: 10.1111/j.1365-2516.2008.01834.x. [DOI] [PubMed] [Google Scholar]
- 27.Bork K, Wulff K, Meinke P, Wagner N, Hardt J, Witzke G. A novel mutation in the coagulation factor 12 gene in subjects with hereditary angioedema and normal C1-inhibitor. Clin Immunol. 2011;141:31–5. doi: 10.1016/j.clim.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Bork K, Wulff K, Hardt J, Witzke G, Lohse P. Characterization of a partial exon 9/intron 9 deletion in the coagulation factor XII gene (F12) detected in two Turkish families with hereditary angioedema and normal C1 inhibitor. Hemophilia. 2014;20:e372–5. doi: 10.1111/hae.12519. [DOI] [PubMed] [Google Scholar]
- 29.Kiss N, Barabas E, Halasz A, Varga AL, Prohanska Z, Szilagyi A. Novel duplication in the F12 gene in a patient with recurrent angioedema. Clin Immunol. 2013;149:142–5. doi: 10.1016/j.clim.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Silverberg M, Dunn JT, Gaven L, Kaplan AP. Autoactivation of human Hageman factor: demonstration utilizing a synthetic substrate. J Biol Chem. 1990;255:7281–6. [PubMed] [Google Scholar]
- 31.Weiss AS, Gallin JI, Kaplan AP. Fletcher factor deficiency: a diminished rate of Hageman factor activation caused by absence of prekallikrein with abnormalities of coagulation, fibrinolysis, chemotactic activity, and kinin generation. J Clin Invest. 1974;53:622–33. doi: 10.1172/JCI107597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tankersley DL, Finlaysen JS. Kinetics of activation and autoactivation of human factor XII. Biochemistry. 1984;23:273–9. doi: 10.1021/bi00297a016. [DOI] [PubMed] [Google Scholar]
- 33.Björkqvist J, de Maat S, Lewandrowski U, Di Gennaro A, Oschatz C, Schönig K, et al. Defective glycosylation of coagulation factor XII underlies hereditary angioedema type III. J Clin Invest. 2015;125:3132–46. doi: 10.1172/JCI77139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuraw BL, Bork K, Binkley KE, Banerji A, Christiansen SC, Castaldo A, et al. Hereditary angioedema with normal C1 inhibitor function: consensus of an international expert panel. Allergy Asthma Proc. 2012;33(suppl):S145–56. doi: 10.2500/aap.2012.33.3627. [DOI] [PubMed] [Google Scholar]
- 35.Kruithof EK, Tran-Thang C, Gudinchet A, Hauert J, Nicoloso G, Genton C, et al. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987;69:460–6. [PubMed] [Google Scholar]
- 36.Kruithof EK, Gudinchet A, Bachmann F. Plasminogen activator inhibitor 1 and plasminogen activator inhibitor 2 in various disease states. Thromb Haemost. 1988;59:7–12. [PubMed] [Google Scholar]
- 37.Kaplan AP, Austen KF. The fibrinolytic pathway of human plasma. Isolation and characterization of the plasminogen proactivator. J Exp Med. 1972;136:1378–93. doi: 10.1084/jem.136.6.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph K, Kaplan AP. Formation of Bradykinin: a major contributor to the innate inflammatory response. Adv Immunol. 2005;86:159–208. doi: 10.1016/S0065-2776(04)86005-X. [DOI] [PubMed] [Google Scholar]
- 39.Miles LA, Greengard JS, Griffin JH. A comparison of the abilities of plasma kallikrein, β-factor XIIa, factor XI and urokinase to activate plasminogen. Thromb Res. 1983;28:407–17. doi: 10.1016/0049-3848(83)90244-x. [DOI] [PubMed] [Google Scholar]
- 40.Smith D, Gilbert M, Owen WG. Tissue plasminogen activator release in vitro in response to vasoactive agents. Blood. 1985;66:835–9. [PubMed] [Google Scholar]
- 41.Brown NJ, Nadean JH, Vaughan DE. Selective stimulation of tissue-type plasminogen activator (tPA) in vivo by infusion of bradykinin. Thromb Haemost. 1997;77:522–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.