Abstract
Cranial ectodermal placodes, which contribute to cranial ganglia and sense organs, share common properties with the neural crest. Both arise from the neural plate border region and share the ability to form peripheral sensory neurons. Although most placodes arise from ectoderm, the olfactory placode arises from the anterior neural folds (ANF), the only region of the neural tube that does not produce neural crest cells. Here, we test the ability of the ANF to form neural crest by performing heterotopic transplantation experiments in the chick embryo. We find that, at the neurula stage (HH stage 7), the chick ANF retains the ability to form migrating neural crest cells when transplanted caudally to rostral hindbrain levels. This ability is gradually lost, such that by HH9, this tissue appears to no longer transfate. In contrast, hindbrain dorsal neural folds transplanted rostrally fail to contribute to the olfactory placode but instead continue to generate neural crest cells. The transcription factor GANF is expressed in the ANF and its morpholino-mediated knock-down expands the neural crest domain rostrally and results in the production of migratory cells emerging from the ANF; however, these cells fail to express the HNK1 neural crest marker, suggesting only partial conversion. Our results show that environmental factors can transfate the chick anterior neural folds to a neural crest cell fate via a mechanism that partially involves loss of GANF.
Introduction
Cranial ectodermal placodes are thickened regions of ectoderm in the head of vertebrate embryos that contribute to the sense organs (nose, ears, lens of the eye) as well as neurons of cranial sensory ganglia. Together with neural crest cells, the ectodermal placodes form the entire peripheral nervous system of vertebrates. Of the cranial placodes, the olfactory placode is one of the most unusual since it forms not from lateral ectoderm but from the anterior neural fold (ANF) (Couly and Le Douarin, 1985). The ANF contains a mixture of precursors for epidermis, olfactory and lens placodes, as well as portions of the forebrain (Bhattacharyya et al., 2004; Cobos et al., 2001) and, interestingly, is the sole region of the neural tube that does not give rise to neural crest cells, which form from the neural folds at all axial levels caudal to the ANF. After its specification at stage 10 (Bhattacharyya and Bronner-Fraser, 2008), the olfactory precursors move to the ventral ectoderm at the level of the forebrain region where the olfactory placode thickens and invaginates to form the olfactory vesicle. A subset of the placode cells subsequently differentiates into olfactory sensory receptor neurons, critical for perception of smell, and project axons to the olfactory bulb in the central nervous system (Buck, 2000). Olfactory sensory neurons are unusual and particularly interesting since they have the rare property of being generated throughout life, even in mammals (Bermingham-McDonogh and Reh, 2011; Graziadei and Graziadei, 1979).
Ectodermal placodes and neural crest share many similar properties. Both originate from neurogenic ectoderm at the border between neural ectoderm and presumptive epidermis (Baker and Bronner-Fraser, 1997a; Webb and Noden, 1993). Both have progeny that undergo an epithelial-to-mesenchymal transition (EMT) and are capable of migration. In addition, they share some common derivatives, e.g. sensory neurons and neuroendocrine cells (Baker and Bronner-Fraser, 1997b). Of the cranial placodes, the olfactory placode shares the most similarities with neural crest cells. Not only is it the sole placode that originates from the anterior neural folds, but like neural crest, it also gives rise to sensory neurons and neuroendocrine cells. These similarities suggest an embryonic and perhaps evolutionary relationship between these two populations.
This raises the intriguing possibility that these two populations may be interchangeable and that environmental factors at different axial levels may play a role in determining whether neural fold cells become olfactory placode or neural crest. To test this, here, we challenge the differentiative ability of the chick ANF to form neural crest when grafted to more caudal levels of the neural axis. These heterotopic grafting experiments demonstrate that ANF cells can undergo a fate transition to a neural crest cell fate switch during a critical period. Molecular analysis suggests that the loss of the transcription factor, GANF, may be partially involved in acquisition of neural crest identity in these cells. These results are consistent with findings in other species suggesting flexibility in ANF fate at early stages (Villaneuva et al., 2002; Carmona-Fontaine et al., 2007; Li et al., 2009).
Materials and Methods
Embryos
Fertilized Colorado Red, White Leghorn chicken eggs, obtained from local farms, were incubated at 38°C for 26–31 h to obtain stage HH7 embryos, or 36–42 h to obtain stage HH 9–10 embryos (Hamburger and Hamilton, 1951). Green Fluorescent Protein (GFP)-transgenic chick eggs were obtained from Dr. Susan Chapman at the University of South Carolina, and used within 3 days of receipt. Transgenic quail eggs were obtained from Dr. Rusty Lansford at Caltech. The Colorado Red and White Leghorn eggs were used as tissue hosts and transgenic chick, electroporated chick, or quail embryos were used as the tissue donors.
Embryos were collected onto Whatman paper rings and placed into sterile Ringers containing 5 mL of Penicillin/Streptavidin per 500 mL of Ringers. The embryos were screened under a fluorescent Leica MZFLIII dissecting microscope, illuminated with an X-cite series 120 fluorescent lamp and a fiber-optic lamp for reflected lighting. All selected donors were transferred into a dish of clean Ringers with penicillin/streptavidin.
Transplants
For transplantation experiments, the anterior neural fold (ANF) of the donor embryo was carefully microdissected with a glass needle. To this end, an incision was made at the anterior tip of the neural fold, followed by a second incision at a distance of about 100 µm posterior to the first. After makig two mediolateral cuts, the tissue was released from the neural fold by scraping carefully along the sides. After dissection, the ANF was pipetted into a glass capillary tube, and set aside.
Host eggs at HH8 (3 to 5 somites) were windowed with a small pair of sharp scissors. A small amount of blue food dye (FD&C Blue, Spectra Color Corp, Kearny NJ) (8 mg/mL food dye in Ringer's solution containing 1% penicillin and streptomycin) was injected sub-blastodermally to aid in visualization. The vitelline membrane was opened with a glass needle. To facilitate experimental manipulation, the embryo was raised to the top of the egg by adding back 3–5 mL of the egg white.
The donor anterior neural fold tissue was pipetted onto the side of the host embryo. A similarly sized opening was made in the host, at the level of the midbrain/rostral hindbrain, by removing and discarding an approximately 100 µm portion of caudal midbrain or rostral hindbrain. The anterior neural fold was carefully inserted into the opening in the host midbrain/rostral hindbrain neural fold, closing it off. After experimental manipulation, a few drops of Ringer's solution was deposited carefully around the embryo, 5–8 mL of egg white were removed to lower the embryo. The egg was sealed with clear adhesive tape and re-incubated at 38°C in a humidified incubator until the host embryo reached HH 13–14. Embryos were fixed into 4% paraformaldehyde overnight at 4°C and rinsed into 3–6 changes of buffer (Phosphate Buffered Saline (PBS) with 0.1% Tween) over a period of 24 hours at 4°C before further processing.
Two types of grafts were performed as positive controls. First, anterior neural folds from stage HH7 donors were transplanted into HH8 control embryos at the level of the anterior neural folds. Secondly, midbrain/hindbrain neural folds from HH7 donors were grafted into HH8 hosts. We used slightly different aged donor and hosts because the embryo develops in an anterior to posterior progression such that the ANF at HH7 is approximately at the same state of development as the hindbrain at HH8. In addition, the HH8 hosts survived the transplantation much better than at earlier stages.
Immunohistochemistry
Embryos were processed for cryosectioning. Briefly, embryos were equilibrated for 1 hour in a 5% sucrose solution, followed by 6 hours to overnight in a 15% sucrose solution. Embryos were next embedded overnight at 37°C in gelatin and sectioned at 10 µm on a MicromHM550 cryostat. Slides were blocked in antibody buffer (PBS+0.1% Tween+5% (by volume) goat serum+ 0.2% BSA) for 1–2 hours at room temperature before the primary HNK1 antibody (hybridoma culture media diluted 1:50 in antibody buffer) was applied overnight at 4°C to recognize migrating neural crest, followed by fluorescently labeled (Alexa 594) secondary antibody for 12 hour at 4°C (1:1000 dilution). Sections were imaged on a Zeiss Axioskop Plus, using the Axiovision (Rel 4.6) software and image processing was done using Adobe Photoshop CS4 Extended Version11.0.
Morpholino and RFP electroporation
Morpholino oligomers (MO) (Gene-Tools, Philomath OR) were prepared in water at 1.5 mM with a double stranded plasmid (ECR1-pTK, 1 mg/ml) serving as a carrier. The MO were electroporated as described previously (Sauka-Spengler and Barembaum, 2008). The control MO was injected on the right ventral side and the GANF MO on the left ventral side of the stage 4–5 embryos ex ovo on filter paper rings in the space between the vitelline membrane and the embryo. The embryos were then electroporated using 5 pulses for 50 ms each at 5.2 V with 100 ms in between each pulse, then incubated at 37°C. After 18 h the embryos were collected and then fixed in paraformaldehyde for 18 h at 4°C and afterwards washed in PBS+0.1% tween then dehydrated in methanol and stored at −20°C. For transplant experiments, the embryos were electroporated on both sides with either the GANF or the control morpholinos, and incubated until they reached stage HH7. The embryos were then used as donors for transplant experiments using non-electroporated embryos as hosts. The sequence of the GANF MO is CTGCACACAGCGATGTACTTGCCAT. The control MO is the Gene-tools standard control MO CCTCTTACCTCAGTTACAATTTATA.
In some cases, donor chick embryos were electroporated with Red Fluorescent Protein (RFP) expression construct into order to label the donor ANF. Electroporations were performed as described above for morpholino and grafting was done as described above.
In situ hybridization
Whole mount in situ hybridization was done using standard protocols (Wilkinson, 1992). The Sox10 construct was kindly provided by Yi-Chuan Cheng. After color development, the two sides of each embryo were compared.
Results
Grafts of labeled anterior neural fold (ANF) to ANF contribute to the olfactory epithelium
According to the fate map of the early avian embryo, the dorsal and most anterior portion of the forming neural tube, the anterior neural fold (ANF) in the chick at stage HH7, contains a mixture of olfactory and lens placode precursors, together with presumptive epidermis and forebrain (Couly and LeDouarin, 1985; Bhattacharyya et al. 2004). These previous results were performed using either quail to chick interspecific grafting (Couly and LeDouarin, 1985) experiments or DiI labeling (Bhattacharyya et al., 2004). To confirm this observation, we performed both interspecific quail to chick grafts as well as chick to chick grafts. For quail grafts, we used transgenic quail that expressed nuclear RFP and for chick grafts, we used transgenic GFP as well as RFP electroporated donor embryos. All three techniques yielded similar results. Figure 1A shows the grafting technique of transplanting the ANF from HH7 into an unlabeled host at HH8 at the same axial level.
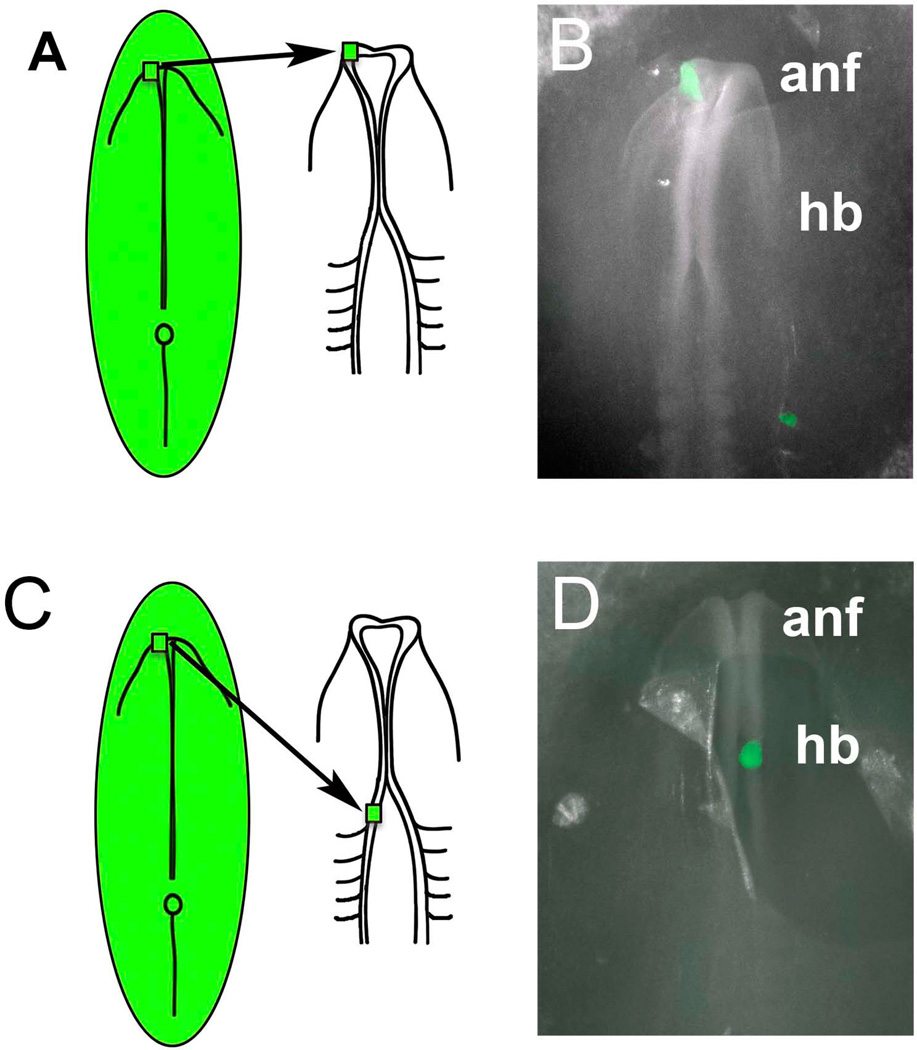
Figure 1.
Schematic diagram illustrating the transplantation paradigm. A) Donor tissue from transgenic GFP chick, transgenic RFP quail or electroporated RFP chick embryos was transplanted from the anterior neural fold (ANF) of the donor embryo into the ANF region of an HH8 host embryo. B) Whole mount image of an embryo after a GFP-transgenic chick graft (green) was transplanted isotopically into the left side of an unlabelled host embryo. C) Donor tissue from transgenic GFP chick, transgenic RFP quail or electroporated RFP chick embryos was transplanted from the anterior neural fold (ANF) of the donor embryo into the rostral hindbrain (HB) region of an HH8 host embryo. D) Whole mount image of an embryo after a GFP-transgenic chick graft (green) was transplanted heterotopically into the right side of an unlabelled host embryo.
Two days post-grafting, we examined the distribution of RFP or GFP cells, viewed in whole mount preparations. The results confirm that these grafts contribute to both the olfactory placode and retina of the eye (Figure 2A–D), consistent with results from previous DiI-labeling (Bhattacharyya et al., 2004) and single cells lineage analysis (Bhattacharyya et al., 2013). Similar results were seen when grafts came from the ANF (n=4) as well as from more ventral regions of the anterior neural tube (n=4).
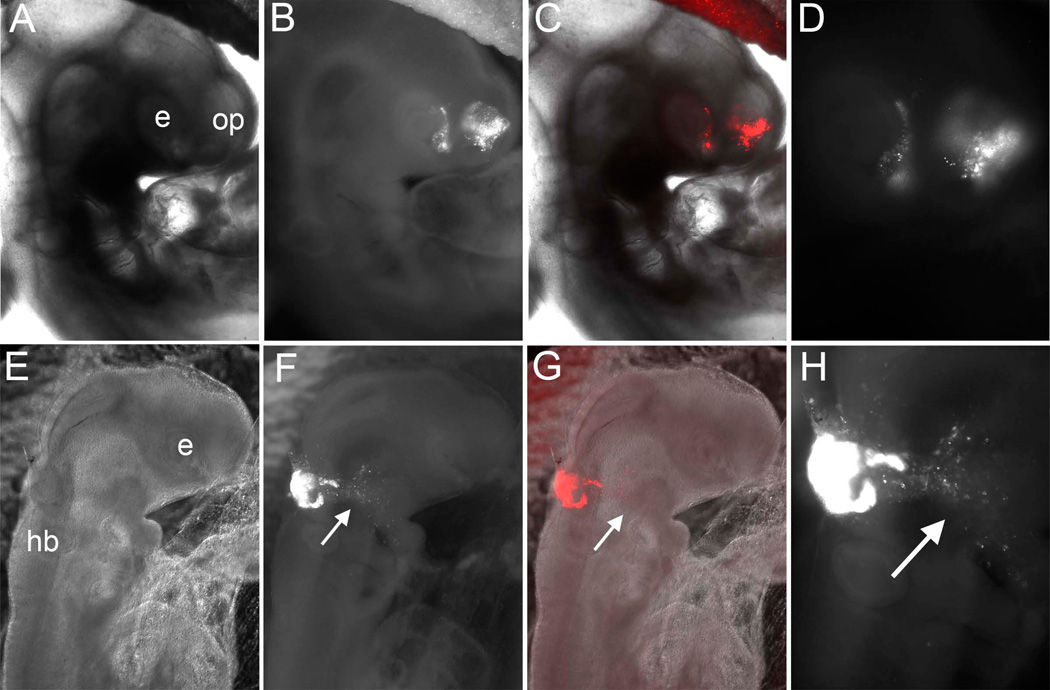
Figure 2.
Results of anterior neural fold (ANF) to ANF transplants (A–D) and ANF to rostral hindbrain (rHB) transplants (E–H) after two days of incubations. A) Bright field, (B, C) Bright field plus fluorescence, and Fluorescence channel alone at higher magnifcation (D) of an embryo two days after grafting an RFP-electroporated ANF in place of the ANF of unlabeled host. Labeled cells populate the olfactory placode epithelium (OP) as well as the eye (E). E) Bright field, (F,G) Bright field plus fluorescence, and Fluorescence channel alone at higher magnification (H) of an embryo two days after grafting an RFP-electroporated ANF into the rostral hindbrain (HB) of an unlabeled host. Labeled cells populate contribute to the hindbrain and also migrate away, as expected for neural crest cells.
When viewed in transverse sections, donor tissue became incorporated into the host ectoderm and olfactory epithelium when viewed at HH14, but transplanted cells failed to contribute to neural crest cells as assayed by HNK-1 staining (Figure 3A–C). Taken together, these results confirm that the ANF can contribute to the olfactory epithelium but not to the neural crest, consistent with previous quail/chick chimeric grafts and DiI labeling studies.
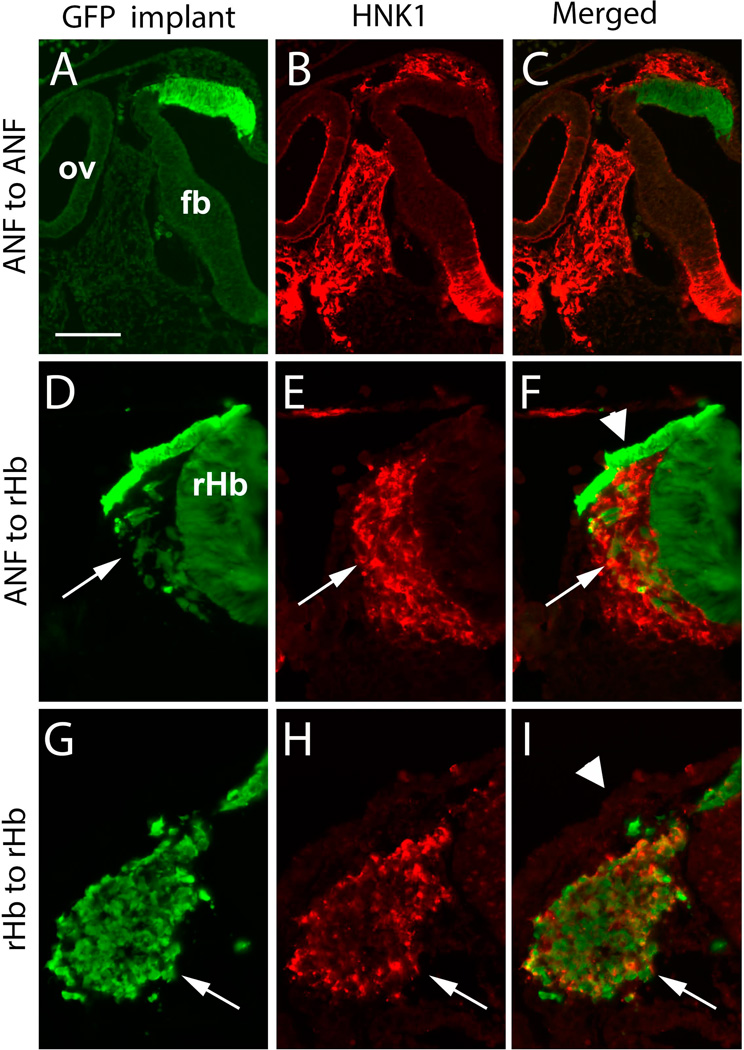
Figure 3.
Transverse sections showing the results of ANF to ANF grafts (A–C), ANF to rostral hindbrain (rHB) grafts (D–F) and rHB to rHB control grafts (G–I). GFP-transgenic chicks were used in all cases shown and sections were stained with HNK1 antibody (red) to identify migrating neural crest cells. A–C) In a section that shows both the olfactory vesicle (OV) and forebrain (FB), the GFP-labeled cells are confined to the forebrain neural tube and/or olfactory region. HNK1-positive neural crest cells have migrated from the neural tube but do not overlap with GFP-expressing cells. (D–F) After transplantation of the ANF to the rostral hindbrain (HB), GFP labeled cells were found in the ectoderm and intermingled with host migrating neural crest cells, with both donor and host cells expressing the HNK1 epitope. Arrows indicate migrating neural crest cells.
Grafts of labeled HH7 ANF to the rostral hindbrain form neural crest cells
We next tested if grafting the ANF to more caudal levels of the neural axis would result in cells differentiating according to their axial level of origin or according to their new location. In this case, donor ANF was transplanted to the level of the rostral hindbrain, as shown in Figure 1B. For chick grafts, we used transgenic GFP as well as RFP electroporated donor embryos and for quail grafts, we used transgenic quail that expressed nuclear Cherry. All grafts yielded similar results, except where indicated, and the data are presented collectively.
Interestingly, the results show that donor ANF grafts from the dorsal neural folds of HH7 embryos transplanted to the level of the rostral hindbrain contribute to the migrating neural crest population after two days of development, as seen in whole mount (Figure 2E–H; n=18). These cells migrate along normal hindbrain neural crest migratory pathways toward the branchial arches. More ventral anterior neural tube grafts (n=6) also produced a small number of neural crest cells but not comparable to dorsal ANF grafts.
In transverse sections, it is clear that donor cells are seen within the migrating HNK-1 stream, intermingling with host neural crest cells (Figure 3D–F) and also contribute to the overlying ectoderm. However, ANF grafts do not contribute as many neural crest cells as do isotopic and isochronic grafts of hindbrain neural folds. A small proportion of cells originating from the ANF grafts migrated out of the grafts but did not express HNK1. In contrast to ANF grafts, isotopic grafts do not contribute to the overlying non-neuronal ectoderm (Figure 3G–I).
We noted some differences between quail and chick grafts. Chick grafts typically contributed many more neural crest cells than quail grafts. In addition, the quail grafts tended to form ectopic vesicles that were never observed in the chick grafts. These results are consistent with previous experiments suggesting differences between quail and chick tissue (Bellairs et al., 1981) and underline the importance of using intraspecific grafts now that transgenic birds are available.
These results suggest that ANF cells at HH7 remain plastic and retain the ability to respond to neural crest inducing signals when removed from their normal environment. However, some ANF cells also contribute to the non-neural ectoderm whereas dorsal neural tube cells from more posterior levels do not. Therefore, some ANF cells behave according to their intrinsic program even after grafting to an ectopic location, reflecting their ability to contribute to epidermis amongst other derivatives.
ANF lose ability to transfate to neural crest over time
The olfactory placode cells become specified by HH10 (Bhattacharyya and Bronner-Fraser, 2008). To examine if the ANF retains the ability to assume a neural crest fate over time, we tested the regulative ability of anterior neural folds to form neural crest from HH8–10 after transplantation to the rostral hindbrain. Whereas HH8 neural folds retained some ability to form neural crest cells (n= 2/3 embryos), this ability was lost by HH9 such that no neural crest cells were observed from grafts at HH9 or later. These results demonstrate that there is a critical period during which the ANF can assume a neural crest fate if experiencing appropriate environment cues.
GANF is expressed in the anterior neural folds
In the mouse, the transcriptional regulator Hesx1 has been shown to be expressed in the anterior neural ectoderm and critical for formation of the anterior forebrain and nasal placode (Martinez-Barbera et al., 2000; 2002). We examined the expression of the chick homolog of Hesx1, called GANF, and find that it is expressed specifically in the ANF at stages HH6–8 (Figure 4A–C), consistent with previous work describing GANF expression in the anterior neural ridge at HH4–HH8 (Sánchez-Arrones et al., 2009). The expression of GANF is maintained in the nasal placode through HH10 (Figure 4D–I), correlating with the time of olfactory placode specification (Bhattacharyya and Bronner-Fraser, 2008) and beyond. We therefore asked if loss of GANF might result in transfating of the ANF to form neural crest cells, mimicking the effects of caudal transplantation.
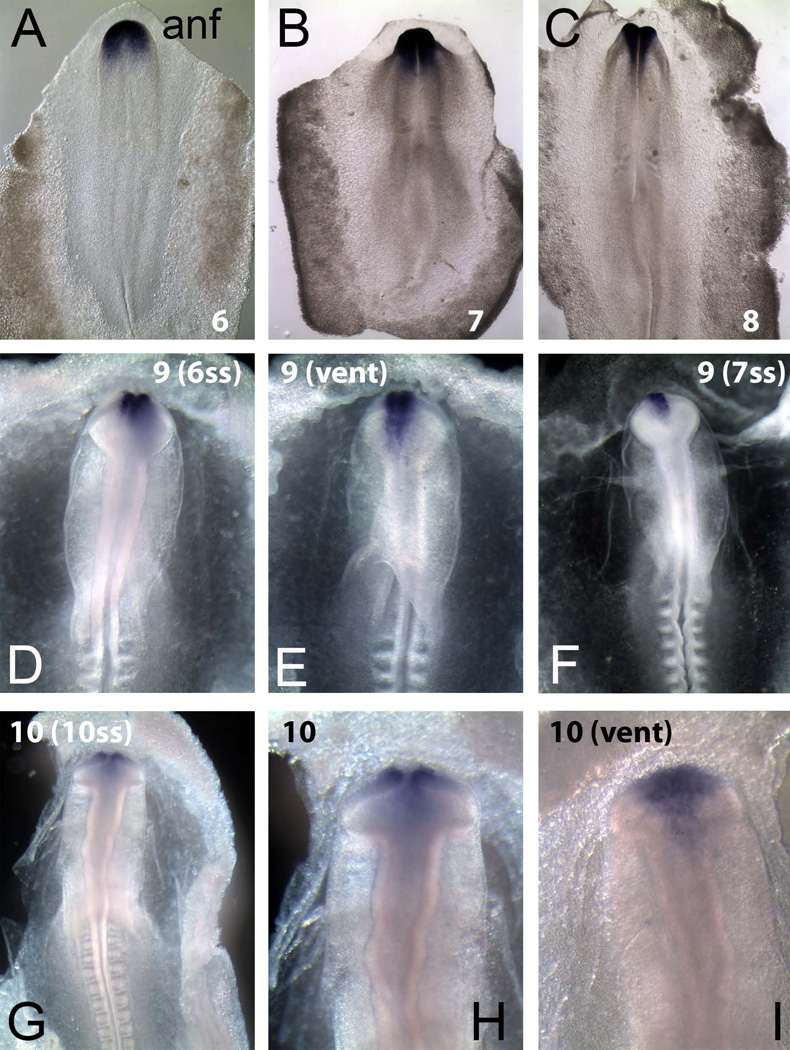
Figure 4.
In situ hybridization showing GANF transcription factor expression in chick embryos from stages HH6–10 in both dorsal and ventral view. (A–C) In dorsal views of HH6–8 embryos, GANF if expressed is consistently expressed in the forming anterior neural folds. (D–F) At HH9, GANF remains expressed in the future olfactory domain as seen in both dorsal and ventral (vent) views. (G–I) At HH10, as the placode moves ventrally, GANF expression correlates well with the olfactory placode domain in both dorsal and ventral views.
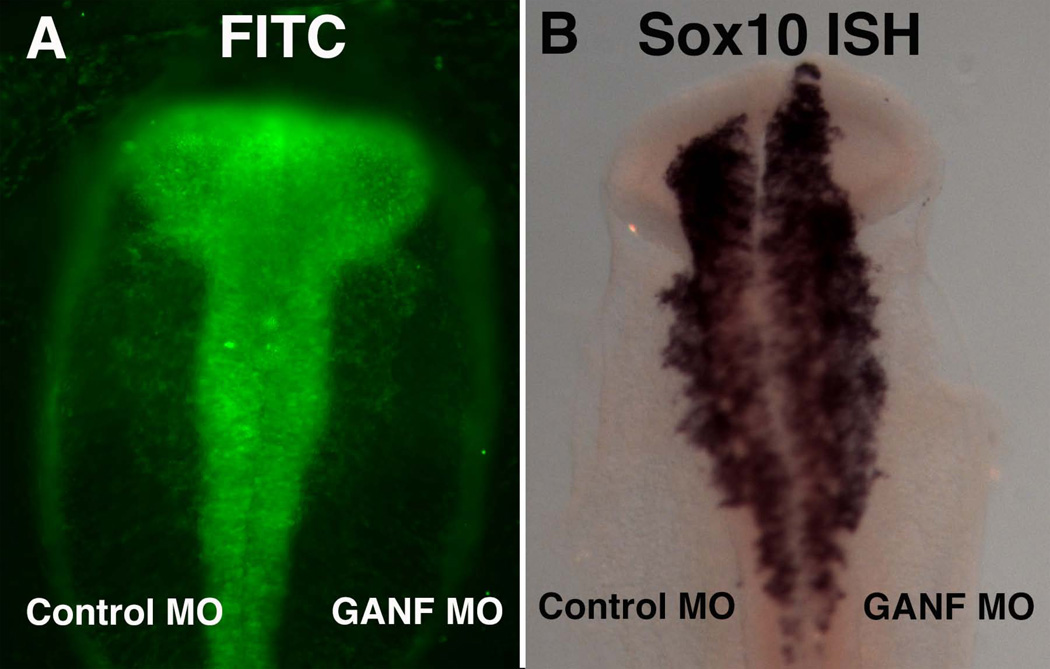
A morpholino designed to block the translational start site of GANF was electroporated onto the right side of HH4 embryos that were then analyzed at HH10 to look at expression of the neural crest marker Sox10. Interestingly, the results show that Sox10 expression is expanded anteriorly in a majority of the electroporated embryos (Figure 5; n = 6/8). This suggests that the neural crest population is enhanced after loss of GANF function in the ANF.
Figure 5.
Morpholino (MO) knock-down of GANF expands causes rostral expansion of the neural crest domain. A) Fluorescence view of embryo electroporated with control MO on the left and GANF MO on the right. Both MOs are conjugated with FITC. B) The same embryo after in situ hybridization with a Sox10 probe to recognize neural crest cells. Note the expansion of Sox10 (arrow) on the right, GANF-MO treated side.
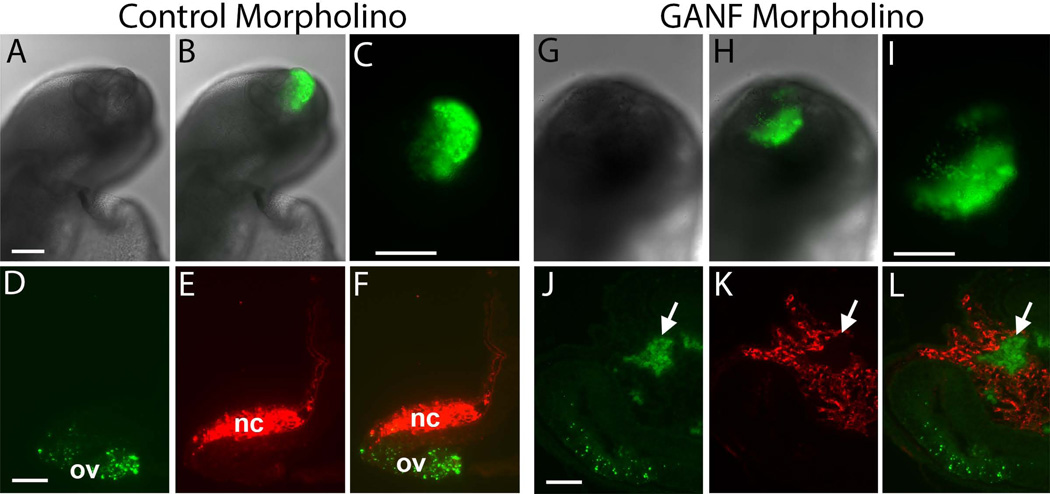
To examine if these neural crest cells are derived from the ANF, we repeated the morpholino (MO) knock-down experiment and incubated GANF (n=6) or control morpholino-treated (n=5) embryos until stage HH7, for use as donors. We then grafted the electroporated ANF into an unlabeled host of similar stage. For both GANF-MO and control-MO grafts, the results show that the FITC labeled ANF cells contribute to the host olfactory placode (Figure 6). After GANF MO treatment, some FITC labeled cells also appeared to have left the ANF and were observed adjacent to and within stream of HNK1-postiive host neural crest cells. However, in no cases did they express HNK1. These results suggest that, although GANF may be important for restricting the neural crest population to caudal locations, and that its loss may aid migration away from the olfactory placode. However, loss of GANF does not appear sufficient to completely transfate the ANF derived cells into neural crest cells, as they failed to upregulate the neural crest marker, HNK1. Therefore, perhaps some other environmental factors present at more caudal levels are necessary for promoting a full transition to the neural crest cell fate.
Figure 6.
Morpholino knock-down of GANF results in some cells migrating away from ANF to ANF grafts. (A–F) An unlabeled host embryo two days after receiving an ANF graft that was electroporated with control FITC (green) morpholino. A) Bright field; B) Bright field plus FITC fluorescence; C) High power of FITC fluorescence; D) Section through the olfactory placode showing that FITC+ cells are confined to the placode; E) HNK1 staining of the same section shows that neural crest (NC) cells are present but not FITC+; F) overlap of FITC and HNK1 staining. (G–L) An unlabeled host embryo two days after receiving an ANF graft that was electroporated with GANF FITC (green) morpholino. G) Bright field; H) Bright field plus FITC fluorescence; I) High power of FITC fluorescence; J) Section through the olfactory placode showing that FITC+ cells are present within the placode but that some cells have also moved away into the periphery; E) HNK1 staining of the same section shows that neural crest (NC) cells are present and intermingled with FITC+ cells but the latter are not HNK1 positive; F) overlap of FITC and HNK1 staining. Green = ANF cells; Red = Host neural crest cells.
Discussion
Neural crest cells and ectodermal placodes have many similarities including origin at the neural plate border region at gastrula stages (Baker and Bronner-Fraser, 1997a; Webb and Noden, 1993), their ability to undergo EMT and migrate, as well as to form some common neuronal and neuroendocrine derivatives (see Baker and Bronner-Fraser, 1997b). Although these common properties have been interpreted to suggest possible embryonic and evolutionary relationships between these two populations, relatively little is known about commonalities underlying their cell fate decisions, induction or differentiation. Whereas the neural crest has been studied in depth, far less is known about the factors involved in induction and maintenance of ectodermal placodes.
Much of our understanding of placodal contributions derives from elegant interspecific quail/chick chimeric studies performed in avian embryos. In these experiments, pieces of ectoderm were grafted orthotopically to study the embryonic origin of various placodes (e.g. D’Amico-Martel and Noden, 1983). The olfactory placode gives rise to the olfactory epithelium, critical for formation of sensory receptor neurons that mediate our sense of smell. Grafting studies in aves have shown that the olfactory placode fate-maps to the anterior neural folds at the 3–4 somite stage (HH7; Couly and Le Douarin, 1985). At later stages, this placode is found in the ventral ectoderm adjacent to the forebrain region. In this study, we repeated the interspecific fate map experiments of Couly and Le Douarin (1985) using both quail/chick and chick/chick grafts to verify that the ANF does in fact contribute to olfactory placode. As expected, the results show that anterior neural folds of HH7 embryos contribute to the olfactory placode and epidermis as well as parts of the forebrain of host embryos.
To examine the competence of the anterior neural folds to form neural crest, we grafted this same ANF tissue to more caudal levels of the midbrain or rostral hindbrain. In this ectopic location, many of the ANF cells became neural crest cells, expressing appropriate markers like HNK1 and migrating away from the graft. Although the factors responsible for converting the ANF into neural crest cells remain unknown, it has been shown that caudal paraxial mesoderm has “caudalizing” activity. For example, markers like Snail2 are induced in anterior neural plate tissue cultured in conjunction with this mesoderm (Muhr et al, 1997). Interestingly, at HH7, this activity appears to be highest in the caudal hindbrain. Thus, it is possible that environmental factors in or adjacent to the midbrain and hindbrain are able to “reprogram” the ANF tissue to assume a neural crest fate.
In contrast to tissue grafted to caudal locations, ANF tissue grafted to an anterior location did not appear to make a complete transition to neural crest cells, even after knock-down of the transcription factor GANF. Although GANF loss of function causes the neural crest domain to expand rostrally and results in some cells migrating away from the graft, its loss was not sufficient to convert the ANF into HNK1-positive neural crest cells. Also, most of the grafted cells remained within the olfactory placode. This may suggest that factors from the immediate environment influence the ANF to remain olfactory. However, these influences do not appear sufficient to block neural crest formation, as we found that rostral hindbrain tissue grafted in place of the ANF continued to produce large numbers of neural crest cells in this ectopic environment.
The proximity of the ANF to the forebrain suggests that the forebrain may play such a role, either inducing or maintaining olfactory placode character. In support of this idea, chick non-neural ectoderm can differentiate into olfactory epithelium in the presence of forebrain tissue (Street, 1937), suggesting that it may have inductive ability. Similarly, in the newt, anterior mesendoderm was shown to be sufficient to elicit olfactory epithelium development in neural plate tissue in co-culture experiments (Jacobson, 1966). One factor in the mesenchyme that has been suggested to contribute to the differentiation of the olfactory epithelium is retinoic acid (La Mantia et al, 1993).
Our results obtained by transplantation in the early chick neurula are consistent with perturbation experiments performed in other species, most notably Xenopus, that suggest that environmental factors can influence the fate of the ANF. In Xenopus, the ANF is competent to form neural crest cells and can be transformed by posteriorizing factors like FGF, Wnt and retinoic acid (Villanueva et al., 2002). Similarly in the chick, activation of canonical Wnt signaling has been shown to expand the neural crest territory into preplacodal regions (Litsiou et al., 2005). Consistent with this, the ANF in Xenopus can be transformed into neural crest by the action of Gbx2, a direct target of Wnt signaling (Li et al., 2009). Reciprocally, neural crest formation in the ANF is actively inhibited by the Wnt antagonist, Dkk1, a mechanism that is conserved from fish to mammals (Carmona-Fontaine et al., 2007). Taken together, this body of work across vertebrates demonstrate that the anterior neural folds have flexibility in prospective fate, at least at early stages.
Adding to these previous observations, the present results demonstrate that there is a critical period, from HH7–8, during which the chick ANF retains the ability to assume neural crest fate when exposed by transplantation to the appropriate environment. In contrast, the rostral hindbrain neural crest at HH7 appears to be specified and no longer inhibited by the ANF environment. We speculate that a combination of neural crest promoting activity in the caudal regions of the embryo and anterior-inducing activity in the forebrain region are likely to create a balance that determines whether neural tube cells can contribute to olfactory epithelium versus neural crest fate.
Acknowledgments
This work was supported by DE16459 to MEB. We thank Elly Chow for their help in early portions of this work and Rusty Lansford for supplying quail embryos.
References
- Baker CVH, Bronner-Fraser M. The origins of the neural crest. Part I: Embryonic induction. Developmental Biology. 1997a;69:3–11. doi: 10.1016/s0925-4773(97)00132-9. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. The origins of the neural crest. Part II: An evolutionary perspective. Developmental Biology. 1997b;69:13–29. doi: 10.1016/s0925-4773(97)00129-9. [DOI] [PubMed] [Google Scholar]
- Bellairs R, Ireland GW, Sanders EJ, Stern CD. The behaviour of embryonic chick and quail tissues in culture. J Embryol Exp Morphol. 1981 Feb;61:15–33. 1981. [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Reh TA. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron. 2011;71:389–405. doi: 10.1016/j.neuron.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Bronner-Fraser M. Competence, specification and commitment to an olfactory placode fate. Development. 2008;135:4165–4177. doi: 10.1242/dev.026633. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A. Pax6, Dlx5 and cell sorting during olfactory and lens placode development: parallels between vertebrates and Drosophila. Devl. Biol. 2004;271:403–414. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Buck LB. The molecular architecture of odor and pheromone sensing in mammals. Cell. 2000;100:611–618. doi: 10.1016/s0092-8674(00)80698-4. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Acuña G, Ellwanger K, Niehrs C, Mayor R. Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Dev. Biol. 2007;309:208–221. doi: 10.1016/j.ydbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Cobos I, Shimamura K, Rubenstein JL, Martínez S, Puelles L. Fate map of the avian anterior forebrain at the four-somite stage, based on the analysis of quail-chick chimeras. Dev. Biol. 2001;239:46–67. doi: 10.1006/dbio.2001.0423. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras I. Dev. Biol. 1985;110:422–439. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras II. Dev. Biol. 1987;120:198–214. doi: 10.1016/0012-1606(87)90118-7. [DOI] [PubMed] [Google Scholar]
- D'Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am. J. Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Graziadei GA, Graziadei PP. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. Journal of Neurocytology. 1979;8:197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Jacobson AG. Inductive processes in embryonic development. Science. 1966;125:25–34. doi: 10.1126/science.152.3718.25. [DOI] [PubMed] [Google Scholar]
- LaMantia AS, Colbert MC, Linney E. Retinoic acid induction and regional differentiation prefigure olfactory pathway formation in the mammalian forebrain. Neuron. 1993;10:1035–1048. doi: 10.1016/0896-6273(93)90052-s. [DOI] [PubMed] [Google Scholar]
- Li B, Kuriyama S, Moreno M, Mayor R. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development. 2009;136:3267–3278. doi: 10.1242/dev.036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Martinez-Barbera JP, Rodriquez TA, Beddington RSP. The homeobox gene Hesx1 is required in the anterior neural ectoderm for normal forebrain formation. Devel. Biol. 2000;223:422–430. doi: 10.1006/dbio.2000.9757. [DOI] [PubMed] [Google Scholar]
- Martinez-Barbera JP, Signore M, Boyl PP, Puelles E, Acampora D, Gogoi R, Schubert F, Lumsden A, Simeone A. Regionalisation of anterior neuroectoderm and its competence in responding to forebrain and midbrain inducing activities depend on mutual antagonism between OTX2 and GBX2. Development. 2002;128:4789–4800. doi: 10.1242/dev.128.23.4789. [DOI] [PubMed] [Google Scholar]
- Sánchez-Arrones L, Ferrán JL, Rodríguez-Gallardo L, Puelles L. Incipient forebrain boundaries traced by differential gene expression and fate mapping in the chick neural plate. Dev. Biol. 2009;335:43–65. doi: 10.1016/j.ydbio.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Barembaum M. Gain- and loss-of-function approaches in the chick embryo. Methods Cell Biol. 2008;87:237–256. doi: 10.1016/S0091-679X(08)00212-4. [DOI] [PubMed] [Google Scholar]
- Street SF. The differentiation of the nasal area of the chick embryo in grafts. J. Exp. Zool. 1937;77:49–80. [Google Scholar]
- Villanueva S, Glavic A, Ruiz P, Mayor R. Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev Biol. 2002;241:289–301. doi: 10.1006/dbio.2001.0485. [DOI] [PubMed] [Google Scholar]
- Webb JF, Noden DM. Ectodermal Placodes: Contributions to the Development of the Vertebrate Head. Amer. Zool. 1993;33:434–447. [Google Scholar]
- Wilkinson DG. Wholemount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In Situ Hybridization: A Practical Approach. Oxford: IRL Press; 1992. pp. 75–83. [Google Scholar]