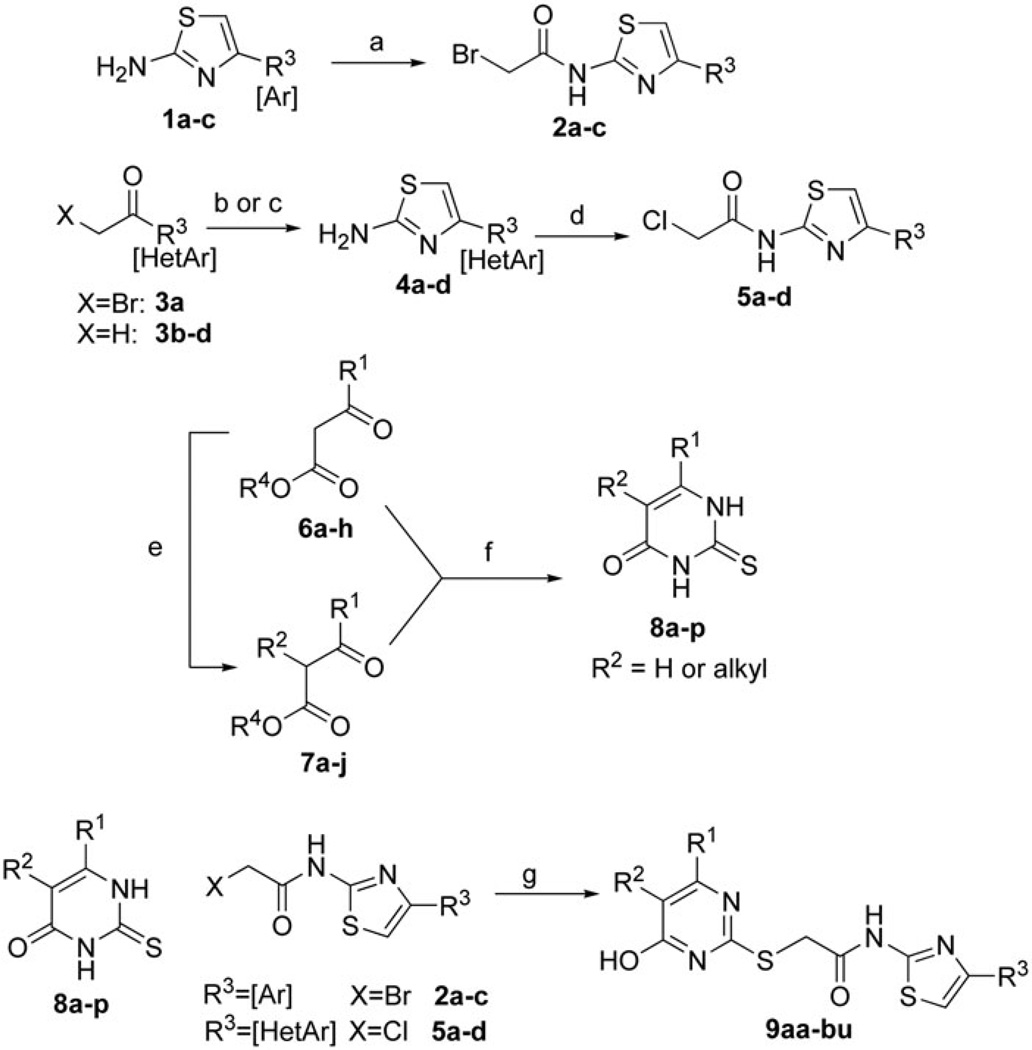
Scheme 1.
Synthesis of 4-aryl/heteroaryl-2-aminothiazole inhibitor candidates. Reagents and conditions: (a) bromoacetyl bromide, Et3N, DCM, 0 °C; (b) for bromoketone 6a: thiourea, THF, 50 °C; (c) for methyl ketones 6b–d: CuBr2, EtOAc, 100 °C; then thiourea 100 °C; (d) bromoacetic acid, EDCI HCl, cat. 4-DMAP, DCE:DMF (1:1), 100 °C; (e) R-X, base, DMF, 60 °C or MW 110 °C (see experimental); (f) Na/EtOH, thiourea, 100 °C; (g) K3PO4-H2O, DMF. For chloroacetamides (X = Cl), NaI was added to facilitate substitution.