FIG 4 .
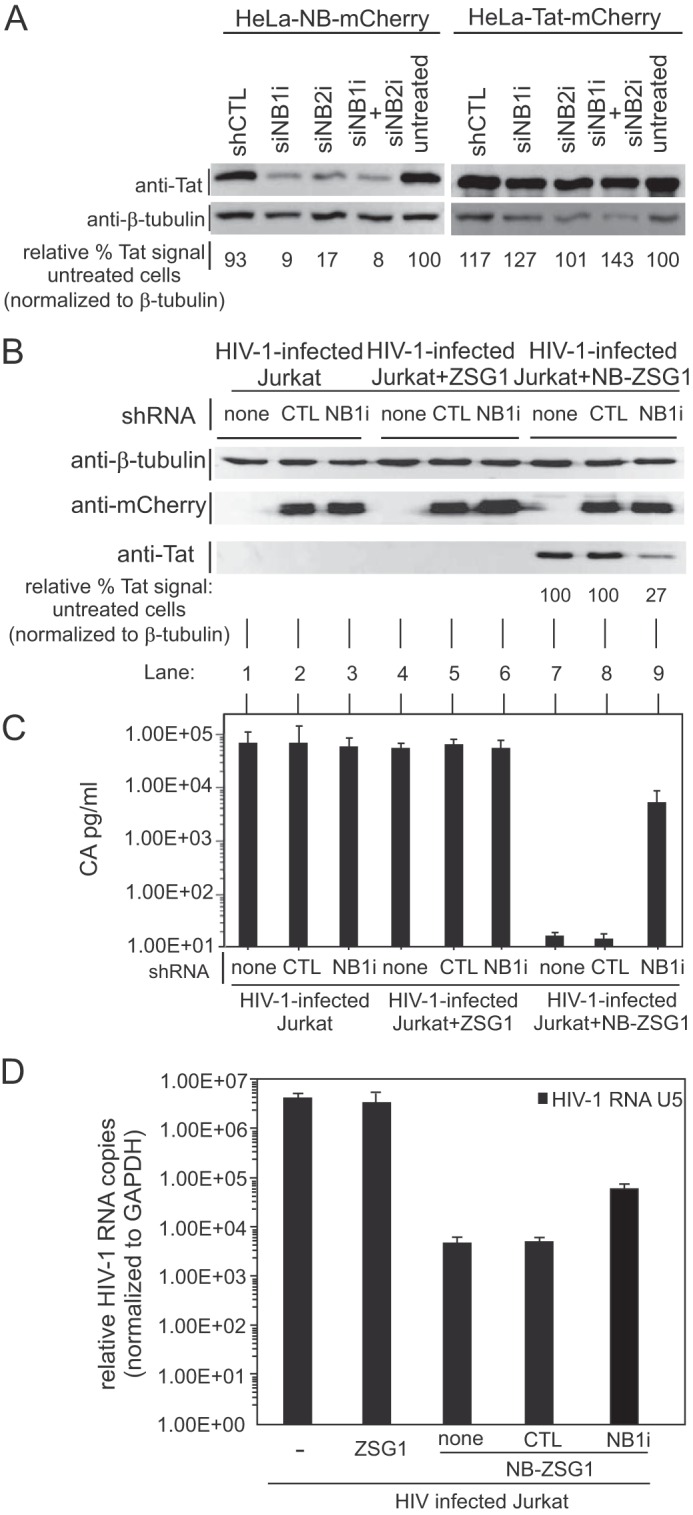
Virus production is restored in NB-ZSG1-treated, HIV-1-infected Jurkat cells by knockdown of NB-ZSG1. (A) Stable HeLa cell lines expressing NB-mCherry or Tat-mCherry were transfected alone or together with two siRNAs (siNB1i and siNB2i) or with a scrambled negative-control (shCTL) siRNA sequence. Cell lysates were analyzed by Western blotting with anti-Tat and anti-β-tubulin antibodies as indicated. A digital image of each Western blot assay was processed with ImageJ software, and the relative Tat signal level was normalized to the β-tubulin signal level in the same sample. (B) Lentiviral vectors that expressed shRNA based on siNB1i or siCTL sequences or the siCTL sequences were used to transduce Jurkat-NB-ZSG1 cells as indicated. Cell lysates were prepared from transduced cells and probed by Western blotting with anti-Tat, anti-mCherry, and anti-β-tubulin antibodies. The relative Tat signal in each sample was measured as described for panel A. (C) The HIV-1 CA present in culture supernatant was quantified by ELISA. Mean values and standard deviations from three independent experiments are shown. (D) Total cellular RNA was isolated from HIV-1-infected Jurkat; NB-ZSG1- or ZSG1-treated, HIV-1-infected Jurkat; or uninfected Jurkat cells. qRT-PCR assays were performed with primers specific for the 5′ UTR. The relative level of viral 5′ UTR RNA in each sample was normalized to the amount of GAPDH mRNA in the sample.
