Figure 2. Regulatory processes impinging on Cdk1 kinase activity.
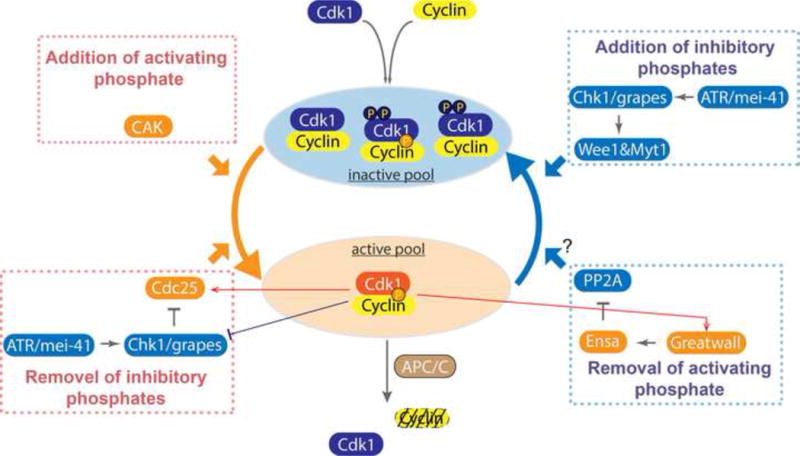
The active form of Cdk1 (in amber oval) has a Cyclin partner, the activating phosphate on the T161 of its T-loop, and is not modified at the inhibitory sites T14 and Y15 whose phosphorylation occludes ATP from the catalytic center. Production of this active form is promoted by the production of Cyclin, the addition of the activating phosphate by Cyclin:Cdk Activating Kinase (CAK), and the Cdc25 dual specificity phosphatase that removes the inhibitory phosphates. Its activity is suppressed by the Wee1 and Myt1 kinases that add inhibitory phosphates, and by destruction of its Cyclin partner in an APC/C stimulated reaction at mitotic exit. Additionally, other important regulators govern the activity of these primary regulators. Protein Phosphatase 2A:B55 (PP2A) counters the action of Cdk1 by removing phosphates from its substrates, and we hypothesis that this phosphatase also removes the activating phosphate from Cdk1 to downregulate its activity. Additionally, the checkpoint kinase Chk1 modulates the activity of the Wee1 kinase and Cdc25 phosphatase to indirectly inhibit activation of Cyclin:Cdk1.
