Abstract
Purpose
To describe outcomes 5 years after initiation of treatment with bevacizumab or ranibizumab for neovascular age-related macular degeneration (AMD).
Design
Cohort study..
Participants
Patients enrolled in the Comparison of AMD Treatments Trials (CATT).
Methods
Patients were randomly assigned to ranibizumab or bevacizumab and to 1 of 3 dosing regimens. After 2 years, patients were released from the clinical trial protocol. At approximately 5 years, patients were recalled for examination.
Main Outcome Measures
Visual acuity (VA) and morphologic retinal features.
Results
VA was obtained for 647 (71%) of 914 living patients with average follow-up time 5.5 years. The mean number of examinations for AMD care after the clinical trial ended was 25.3, and the mean number of treatments in the study eye was 15.4. Most (60%) patients were treated ≥1 times with a drug other than their randomly assigned drug. At the 5-year visit, 50% of study eyes had VA 20/40 or better and 20% had VA 20/200 or worse. Mean change in VA was −3 letters from baseline and −11 letters from 2 years. Among 467 eyes with fluorescein angiography, mean total lesion area was 12.9 mm2, a mean of 4.8 mm2 larger than at 2 years. Geographic atrophy was present in 213 (41%) of 515 gradable eyes and was subfoveal in 85 (17%). Among 555 eyes with spectral domain optical coherence tomography, 83% had fluid (61% intraretinal, 38% subretinal, and 36% sub-retinal pigment epithelium). Mean foveal total thickness was 278 μm; a decrease of 182 μm from baseline and 20 μm from 2 years. An abnormally thin retina at the foveal center (<120 μm) was present in 36%. Between 2 and 5 years, the group originally assigned to ranibizumab for 2 years lost more VA than the bevacizumab group (−4 letters; p=0.008). Otherwise, there were no statistically significant differences in VA or morphological outcomes between drug or regimen groups.
Conclusion
Vision gains during the first 2 years of the trial were not maintained at 5 years. However, 50% of eyes had VA 20/40 or better, confirming anti-VEGF therapy as a major long-term therapeutic advance for neovascular AMD.
Anti- vascular endothelial growth factor (VEGF) therapy has revolutionized the treatment of neovascular age-related macular degeneration (AMD). Currently, nearly all patients diagnosed with neovascular AMD are treated with intravitreal administration of drugs that target VEGF. In 2005 and 2006, results from Phase III randomized clinical trials showed dramatic improvements in visual acuity when eyes with neovascular AMD were treated with ranibizumab (Lucentis) compared to sham treatment or photodynamic therapy.1,2 During the 1-year period between first presentation of results and approval of ranibizumab by the Food and Drug Administration, ophthalmologists began treating neovascular AMD patients with off-label bevacizumab (Avastin). Despite the absence of evidence of efficacy and safety from any randomized clinical trials, use of bevacizumab moved quickly from rescue therapy to first-line therapy.3–5 Subsequently, large-scale, multicenter randomized clinical trials of ranibizumab and bevacizumab were initiated in the United States and 5 other countries to compare safety and effectiveness.6–14 Results from these trials showed that visual acuity outcomes at 1 and 2 years were similar between ranibizumab and bevacizumab under several different dosing strategies. A recent meta-analysis of all comparative trials yielded essentially no difference between drugs in mean change in visual acuity at 1 year (bevacizumab-ranibizumab, −0.5 letters, 95% confidence interval [−1.6, 0.6]).15 Results from later Phase III clinical trials showed that aflibercept (Eylea) injected every 8 weeks provided gains in visual acuity equivalent to those of ranibizumab injected every 4 weeks.16–17
Although clinical outcomes from the first 1 to 2 years of anti-VEGF treatment have been well documented by large-scale clinical trials, relatively few investigators have addressed outcomes after 4 or more years.18–24 Longer term outcomes that have been reported vary considerably across studies. In addition, the annual number of treatments has been low in some reports and only patients who continued regular follow-up and treatment have been included in other reports. In this paper, we report the clinical outcomes of patients enrolled in the Comparison of AMD Treatments Trials (CATT) who returned at approximately 5 years after initiation of treatment with either ranibizumab or bevacizumab. The clinical trial ended after 2 years of follow-up when patients were released from the study protocol. All CATT patients who were alive at the end of the clinical trial were targeted for participation in the CATT Follow-up Study.
METHODS
Design of the CATT Clinical Trial
The design and methods for the clinical trial have been published; therefore, only the key features with bearing on this paper are provided.6,7,25,26 Patients enrolled in CATT between February 20, 2008 and December 9, 2009. Eligible eyes (one study eye per patient) had active choroidal neovascularization secondary to AMD, no previous treatment, visual acuity between 20/25 and 20/320, and neovascularization, fluid, or hemorrhage under the foveal center. Patients were assigned randomly to 1 of 4 treatment groups defined by drug (ranibizumab or bevacizumab) and by dosing regimen (monthly or as-needed [PRN]). At one year, patients initially assigned to monthly treatment were re-assigned randomly to either monthly or PRN treatment (“switched regimen group”). A volume of 0.05 ml containing either 0.50 mg ranibizumab or 1.25 mg bevacizumab was used for intravitreal injection. Patients on the PRN dosing regimen were evaluated for treatment every 4 weeks and treated when fluid on optical coherence tomography (OCT), new or persistent hemorrhage, decreased visual acuity relative to the previous visit, or dye leakage on fluorescein angiography was present. All patients were scheduled for follow-up visits every 4 weeks through 104 weeks. Patients were released from their assigned treatment groups during the visit at 104 weeks; at that visit and thereafter, all treatments were administered according to best medical judgment. The study was registered (NCT00593450) on http:/www.clinicaltrials.gov.
Follow-up Methods
All patients who enrolled in the clinical trial, except for those known to be dead at 2 years, were targeted for participation in the Follow-up Study. Clinical coordinators attempted to contact patients and schedule an appointment for them to be seen in a CATT clinical center between March 14, 2014 and March 31, 2015. Patients completing a visit in a CATT clinical center signed a consent statement for the follow-up visit and signed a medical records release form if they had received care for AMD from outside the CATT clinical center. Patients were interviewed about treatment to either eye, visits to ophthalmologists, and serious medical events since their last visit in the clinical trial. Returning patients had a dilated eye examination, refraction and visual acuity measurement, spectral domain OCT, fundus color stereophotography and fluorescein angiography. All examinations were performed by study-certified personnel following the same protocols used during the clinical trial. Some patients who did not complete a visit in a CATT clinical center were willing to complete an interview about past care, treatment, and serious medical events and/or signed a medical records release form. Information on treatment, visual acuity, and imaging was requested from the outside ophthalmologists who provided AMD care for these patients. The institutional review board associated with each of the participating CATT centers reviewed and approved the Follow-Up study protocol and consent forms. The study was performed in compliance with the Health Insurance Portability and Accountability Act and adhered to the tenets of the Declaration of Helsinki.
When patients were unable or refused to participate, could not be contacted, or were identified as deceased, clinical coordinators submitted patient status forms to the CATT Coordinating Center. After the end of the recruitment period, information on patients whose life status could not be verified and on patients reported as deceased, but without confirmation of the cause of death, was submitted to the National Death Index. When a match was identified (≥99% chance of being correct), the date and cause of death were returned to the CATT Coordinating Center for use in data analysis.
Ascertainment of History of Patient Care and Treatment
Medical records from the CATT clinical center were abstracted for the date of each visit for AMD care at the center after the clinical trial ended, dates of treatment for either eye, and type of treatment administered (bevacizumab, ranibizumab, aflibercept, pegaptanib (Macugen), triamcinolone, photodynamic therapy, thermal laser, and any other treatment). When patients reported care from outside of the CATT center and signed a medical records release form, the same information was requested from each ophthalmologist who provided AMD care for the patient.
Data and Statistical Analysis
Only patients with a visual acuity measurement between 51 months (4.3 years) and 85 months (7.1 years) after the date of treatment assignment in the clinical trial were included in the data analyses, tables, and graphs on outcomes presented in this paper. The limits of the interval represent the minimum and maximum times between the enrollment period for the clinical trial and the enrollment period for the Follow-up Study. Differences in outcomes between drugs and among dosing regimens were assessed with analysis of variance for continuous outcome measures and chi-square tests for categorical outcome measures. Retinal thickness was classified as above (>212μ) or below (<120μ) 2 standard deviations from the mean of normal eyes.27 Serious medical events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) system and further classified as arteriothrombotic and as previously associated with drugs affecting the VEGF pathway (arteriothrombotic events, systemic hemorrhage, congestive heart failure, venous thrombotic events, hypertension, vascular death).28–30 Investigators from 3 of the 43 CATT clinical centers chose not to participate in the Follow-up Study; the 27 patients from these centers were considered non-participants and excluded from the analyses on serious medical events. Statistical computations were performed with SAS 9.4.
RESULTS
Patients
Among the 1117 patients alive at the end of the clinical trial (end of Year 2), 203 (18.2%) died before the end of the Follow-up Study. Of the remaining 914 patients, 647 (70.8%) had a visual acuity measurement in the required time interval of 51 months (4.3 years) to 85 months (7.1 years) after assignment of treatment in the clinical trial. The mean (SD) time interval between enrollment in the clinical trial and the Follow-up Study visit was 66.5 (6.7) months (5.5 years). The percentage of patients with a visual acuity measurement was similar across the 6 drug-dosing regimen groups, ranging from 68.3% to 75.0%. Most (85.5%) of the visual acuity information was obtained by examination at a CATT clinical center by a certified examiner. Three CATT centers responsible for 27 (3.0%) patients did not participate in the Follow-up Study. Forty-one patients (4.5%) agreed to be interviewed but had no visual acuity information available, 102 (11.1%) declined participation, 93 (10.2%) could not be contacted, and 4 (0.4%) could not provide informed consent because of dementia or the absence of a consent statement in their native language.
The characteristics at baseline and at 2 years of the patients who participated in the Follow-up Study are displayed in Table 1, along with characteristics of those who did not participate and those who died after their 2-year visit. Non-participants had a mean age 2.3 years older (p<0.001) and a mean baseline visual acuity score 3.1 letters worse than participants (p=0.001). At 2 years, non-participants had a mean visual acuity 5.4 letters worse (p<0.001) than participants. Among patients assigned treatment PRN for 2 years, non-participants had a mean 1.8 fewer injections (p=0.01). Patients who died after 2 years were on average 5.6 years older than participants and had worse mean visual acuity both at baseline (−4.1 letters) and 2 years (−7.9 letters). Baseline ocular characteristics were similar among these 3 groups of patients.
Table 1.
Characteristics at Baseline and 2 Years by Participation in the Follow-up Study
| Characteristics at Baseline | Participants (N=647) | Non-participants (N=267) | P-value* | Died after Clinical Trial (N=203) |
|---|---|---|---|---|
| Age (yrs); mean (SD) | 77.5 (7.3) | 79.8 (7.8) | <0.001 | 83.0 (6.1) |
| Female gender; no. (%) | 419 (64.8%) | 166 (62.2%) | 0.46 | 112 (55.2%) |
| White race; no. (%) | 637 (98.5%) | 261 (97.8%) | 0.63 | 202 (99.5%) |
| Never cigarette smoking; no. (%) | 274 (42.3%) | 136 (50.9%) | 0.06 | 80 (39.4%) |
| Definite hypertension; no. (%) | 438 (67.7%) | 187 (69.7%) | 0.73 | 152 (74.9%) |
| Visual acuity score, letters; mean (SD) | 62.2 (13.1) | 59.1 (13.6) | 0.002 | 58.0 (13.5) |
| Total area of neovascular lesion (mm2); | 6.4 (6.6) | 6.1 (5.7) | 0.60 | 6.3 (7.0) |
| Geographic atrophy; no. (%) | 47 (7.3%) | 17 (6.4%) | 0.64 | 13 (6.4%) |
| Total retinal thickness; mean (SD) | 464 (185) | 469 (212) | 0.73 | 447 (168) |
| Characteristics at 2 Years | ||||
| Completed Year 2 visit | 643 | 221 | 170 | |
| Visual acuity score, letters; mean (SD) | 69.7 (16.6) | 64.3 (19.5) | <0.001 | 61.9 (21.0) |
| Total area of neovascular lesion (mm2) | 8.1 (7.9) | 8.3 (7.6) | 0.80 | 8.9 (8.6) |
| Geographic atrophy; no. (%) | 127 (19.9%) | 52 (24.9%) | 0.13 | 37 (22.4%) |
| Scarring; no. (%) | 280 (44.2%) | 78 (37.7%) | 0.10 | 69 (41.8%) |
| Total retinal thickness; mean (SD) | 301 (145) | 292 (149) | 0.43 | 280 (123) |
| Number of injections – eyes | ||||
| assigned as-needed dosing yrs; mean (SD) for 2 | 13.3 (6.8) | 11.5 (7.1) | 0.01 | 12.7 (6.7) |
| N | 326 | 134 | 98 | |
P-values are for the comparison of participants to non-participants
Care and Treatment after Release from the Clinical Trial Protocol
Most (591 [91.3%]) of the 647 Follow-up Study patients continued care at a CATT center after release from the clinical trial; however, 51 (7.9%) were seen also or seen exclusively by non-study ophthalmologists, and 5 (0.8%) received no eye care. Records were obtained for 49 (96%) of the 51 patients seen by non-study ophthalmologists. The mean (SD) number of visits for AMD care between the end of the clinical trial and the Follow-up Study visit was 25.3 (13.3); with 8.0 (4.0) in Year 3, 7.2 (4.0) in Year 4, and 6.5 (4.0) in Year 5. The mean (SD) number of treatments was 15.4 (12.5) with 4.8 (4.0) in Year 3, 4.5 (3.8) in Year 4, and 4.0 (3.6) in Year 5. The most recent treatment in the study eye before the Follow-up Study visit was within 3 months for 360 (55.6%) patients. There were 96 (14.8%) patients who had no treatments between the end of the clinical trial and the Follow-up Study visit, with a mean (SD) of 12.5 (8.4) visits. Among these 96 patients, 21 (48.8%) of 43 patients treated PRN in Year 2 of the clinical trial received no treatment during Year 2.
After release from the clinical trial protocol, more than half of the patients received a treatment other than the drug assigned to them in the clinical trial. Among the 328 patients assigned to ranibizumab, 46 (14.0%) had no treatments, 64 (19.5%) had treatments with only ranibizumab, and 218 (66.5%) had at least 1 other type of treatment (Table 2). Among the 319 patients assigned bevacizumab, 50 (15.7%) had no treatments, 99 (31.0%) had treatments with only bevacizumab, and 170 (53.3%) had at least 1 other type of treatment.
Table 2.
Drugs Used to Treat the Study Eye after the End of the Clinical Trial
| Drugs Used | Drug Assigned in the Clinical Trial | |
|---|---|---|
| Ranibizumab (N=328) | Bevacizumab (N=319) | |
| None | 46 (14.0%) | 50 (15.7%) |
| Bevacizumab only | 77 (23.5%) | 99 (31.0%) |
| Ranibizumab only | 64 (19.5%) | 37 (11.6%) |
| Aflibercept only | 8 (2.4%) | 4 (1.3%) |
| Bevacizumab and ranibizumab | 41 (12.5%) | 31 (9.7%) |
| Bevacizumab and aflibercept | 28 (8.5%) | 35 (11.0%) |
| Ranibizumab and aflibercept | 36 (11.0%) | 28 (8.8%) |
| Bevacizumab, ranibizumab and aflibercept | 28 (8.5%) | 33 (10.3%) |
| Other treatment | 0 (0.0%) | 2 (0.6%) |
Visual Acuity
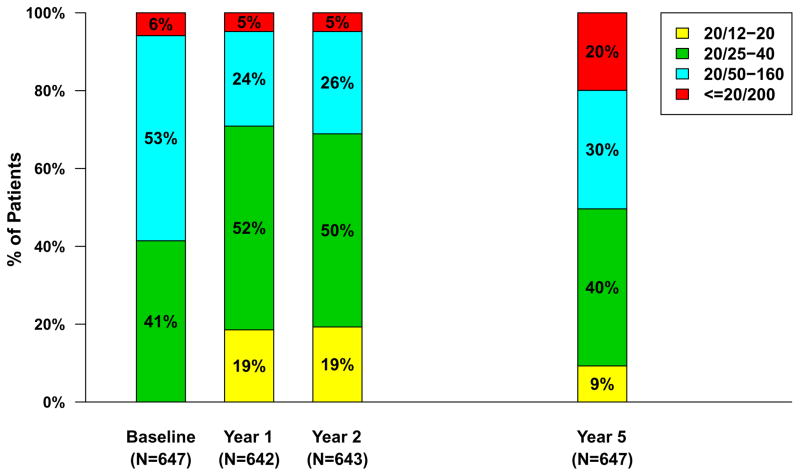
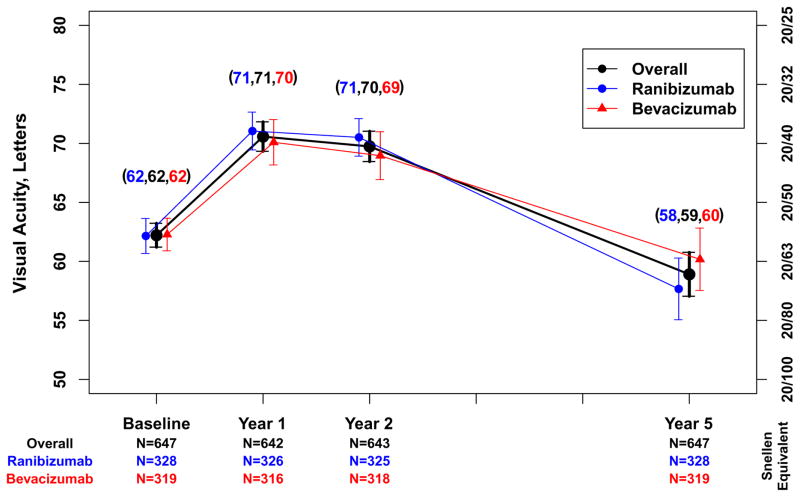
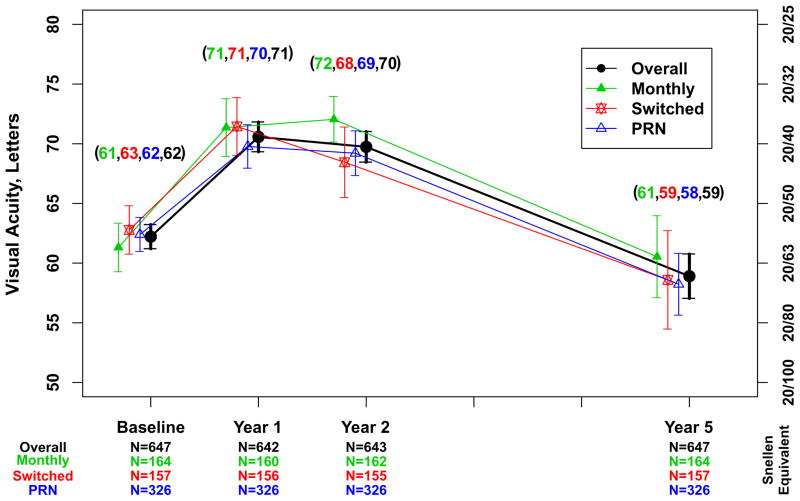
Approximately half (321 [49.6%]) of the 647 Follow-up Study patients had visual acuity 20/40 or better at approximately 5 years (Table 3). The percentage of eyes with visual acuity 20/200 or worse was 5% to 6% at baseline through 2 years and increased to 20% by the Follow-up Study visit (Figure 1). The mean (SD) visual acuity score was 58.9 [20/63] (24.1) letters (Figure 2A). The mean (SD) change from Year 2 was −10.8 (18.9) letters and the mean change from baseline was −3.3 (22.3) letters. Mean visual acuity was similar among eyes assigned to ranibizumab (57.7 letters) and eyes assigned to bevacizumab (60.2 letters; p=0.19) throughout the first 2 years in the clinical trial. Relative to the mean visual acuity at 2 years, eyes assigned to ranibizumab had lost more letters at the Follow-up Study visit (−12.7) than eyes assigned to bevacizumab (−8.8; p=0.008) (Figure 2A). There were no statistically significant differences in these vision outcomes among eyes assigned to the different dosing regimens (Table 4 [available at http://www.aaojournal.org] ; Figure 2B).
Table 3.
Vision and Morphological Outcomes for All Eyes and by Drug Assigned in the Clinical Trial
| Outcome | All (N=647) | Drug Assigned in the Clinical Trial | P-value | ||||
|---|---|---|---|---|---|---|---|
| Ranibizumab (N=328) | Bevacizumab (N= 319) | ||||||
| Visual acuity score, letters | |||||||
| Snellen equivalent, no. (%) | |||||||
| 83–97, 20/12–20 | 60 | ( 9.3) | 24 | ( 7.3) | 36 | (11.3) | |
| 68–82, 20/25–40 | 261 | (40.3) | 129 | (39.3) | 132 | (41.4) | |
| 53–67, 20/50–80 | 132 | (20.4) | 68 | (20.7) | 64 | (20.1) | |
| 38–52, 20/100–160 | 65 | (10.0) | 36 | (11.0) | 29 | ( 9.1) | |
| 37–18, 20/200–400 | 73 | (11.3) | 42 | (12.8) | 31 | ( 9.7) | |
| ≤17, ≤20/400 | 56 | ( 8.7) | 29 | ( 8.8) | 27 | ( 8.5) | |
| Mean letters (SD) | 58.9 | (24.1) | 57.7 | (42.1) | 60.2 | (24.1) | 0.19 |
| Change in visual acuity score, | |||||||
| from baseline, letters, no. (%) | |||||||
| ≥15 increase | 114 | (17.6) | 49 | (14.9) | 65 | (20.4) | |
| 5–14 increase | 156 | (24.2) | 76 | (23.2) | 80 | (25.0) | |
| ≤ 4 change | 142 | (21.9) | 76 | (23.2) | 66 | (20.7) | |
| 5–14 decrease | 82 | (12.7) | 48 | (14.6) | 34 | (10.7) | |
| 15–29 decrease | 71 | (11.0) | 37 | (11.3) | 34 | (10.7) | |
| ≥30 decrease | 82 | (12.7) | 42 | (12.8) | 40 | (12.5) | |
| Mean (SD) | -3.3 | (22.3) | -4.5 | (22.3) | -2.1 | (22.3) | 0.17 |
| Change in visual acuity score, | |||||||
| from 2 years, letters, no. (%)§ | |||||||
| ≥15 increase | 17 | ( 2.6) | 5 | ( 1.5) | 12 | ( 3.8) | |
| 5–14 increase | 60 | ( 9.3) | 28 | ( 8.6) | 32 | (10.0) | |
| ≤ 4 change | 215 | (33.4) | 101 | (31.1) | 114 | (35.8) | |
| 5–14 decrease | 167 | (26.0) | 90 | (27.7) | 77 | (24.2) | |
| 15–29 decrease | 101 | (15.7) | 48 | (14.8) | 53 | (16.7) | |
| ≥30 decrease | 83 | (12.9) | 53 | (16.3) | 30 | ( 9.4) | |
| Mean (SD) | -10.8 | (18.9) | -12.7 | (19.4) | -8.8 | (18.2) | 0.008 |
| Total thickness at fovea, μm | (N=555) | (N=277) | (N=278) | ||||
| Mean (SD)* | 278 | (160) | 267 | (145) | 289 | (174) | 0.11 |
| Mean Change(SD) from 2 years*** | -20 | (132) | -23 | (129) | -17 | 136 | 0.63 |
| Fluid on optical coherence tomography | |||||||
| None | 94 | (17.0) | 42 | (15.3) | 52 | (18.8) | 0.27 |
| Present | 458 | (83.0) | 233 | (84.7) | 225 | (81.2) | |
| Unknown/missing | 3 | 2 | 1 | ||||
| Dye leakage on angiogram | ((N**=527/467) | (N**=265/228) | (N**=262/239) | ||||
| None | 342 | (75.5) | 167 | (75.6) | 175 | (75.4) | 0.97 |
| Present | 111 | (24.5) | 54 | (24.4) | 57 | (24.6) | |
| Unknown/missing | 74 | 44 | 30 | ||||
| Area of lesion, mm2 | |||||||
| Mean (SD)‡ | 12.9 | (11.4) | 13.9 | (11.7) | 11.9 | (11.0) | 0.06 |
| Mean Change (SD) from 2years‡‡ | 4.8 | (8.8) | 5.6 | (9.9) | 4.2 | (7.6) | 0.10 |
| Geographic atrophy, no. (%) | |||||||
| None | 302 | (58.6) | 145 | (55.8) | 157 | (61.6) | 0.34 |
| Non-foveal | 128 | (24.9) | 67 | (25.8) | 61 | (23.9) | |
| Foveal | 85 | (16.5) | 48 | (18.5) | 37 | (14.5) | |
| Unknown/missing | 12 | 5 | 7 | ||||
4 Year 2 visual acuity scores missing; 3 in ranibizumab and 1 in bevacizumab group
3 missing in total thickness at Year 5; 2 in ranibizumab and 1 in bevacizumab
Number with color photographs/fluorescein angiograms
8 missing in change of total thickness from Year 2; 6 in ranibizumab and 2 in bevacizumab group
34 with lesion area ungradable at Year 5; 15 ranibizumab and 19 in bevacizumab group
53 with missing in change of area of lesion due to ungradable images at Year 2 or Year 5;, 29 in ranibizumab and 24 in bevacizumab group
Figure 1.
Distribution of visual acuity over time for 647 patients in the Follow-up Study.
Figure 2.
Mean visual acuity and 95% confidence interval for 647 patients in the Follow-up Study. A. Overall and by drug assigned in the clinical trial; B. Overall and by dosing regimen assigned in the clinical trial.
Morphologic Outcomes from OCT
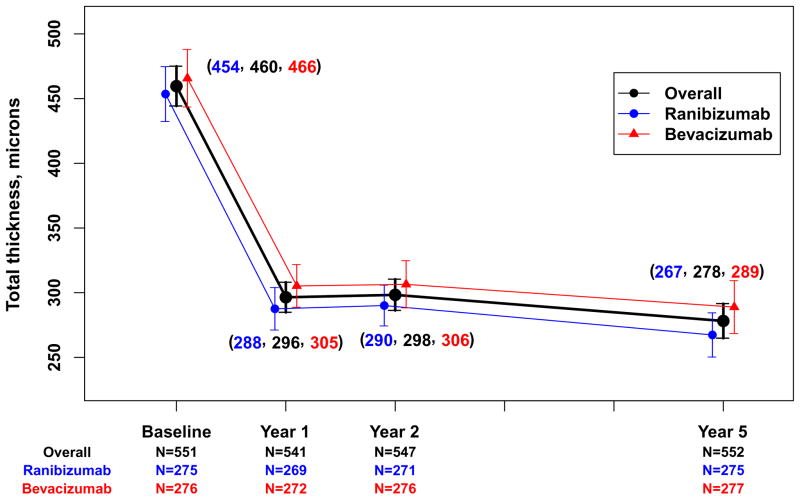
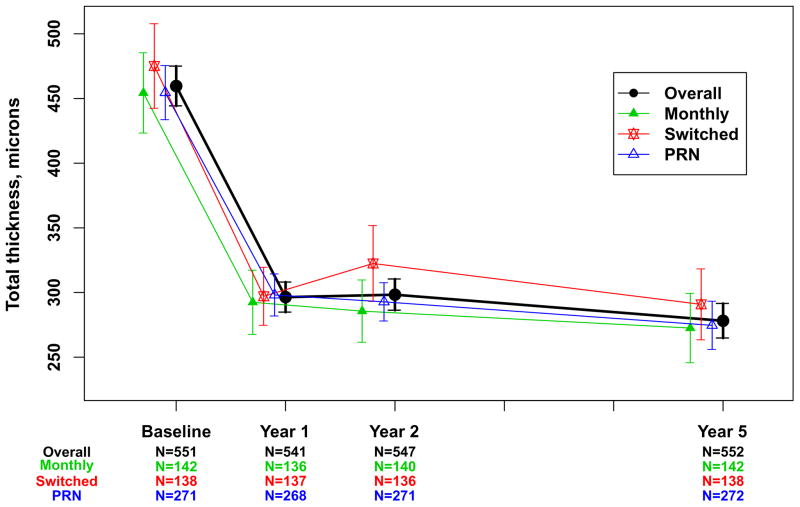
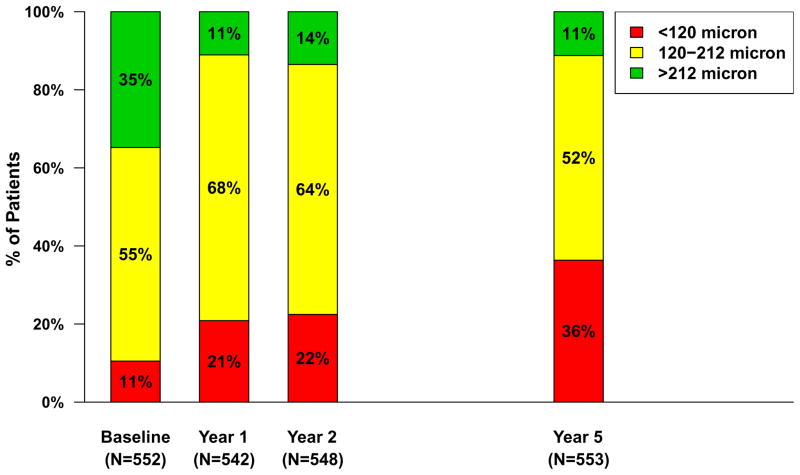
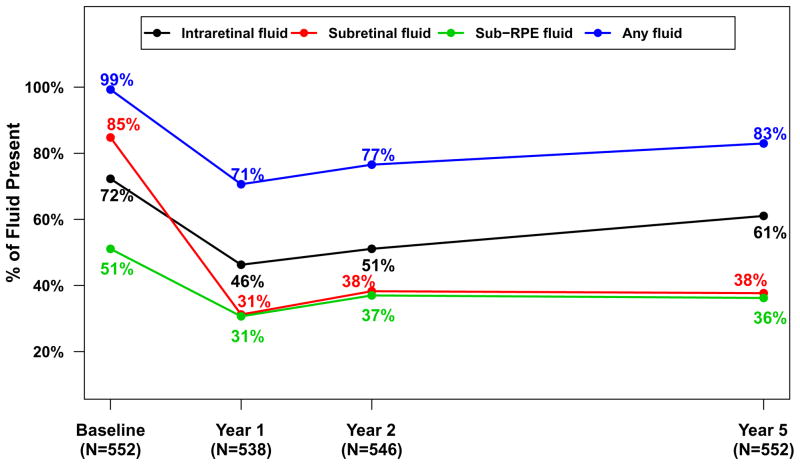
Spectral domain OCT scans were available for 555 (85.8%) of the Follow-up Study patients (Table 3). The mean (SD) total thickness at the foveal center was 278 (160) μm corresponding to a mean change from 2 years of −20 (132) μm (Table 3) and a mean change of −182 (209) μm from baseline (Figure 3A). Neurosensory retinal thickness was less than 120 μm in 201 (36.2%) eyes, an increased percentage from 22% at 2 years (Figure 4). Retinal thickness was greater than 212 μm in 62 (11.2%) eyes, similar to the percentage (14%) at 2 years. Intraretinal, subretinal, or sub-retinal pigment epithelium fluid was present in 458 (83.0%) of 552 gradable eyes (Figure 5). Although the percentages with subretinal fluid (37.7%) and sub-retinal pigment epithelium fluid (36.2%) at 5 years were similar to the percentages at Year 2, the percentage with intraretinal fluid (61.0%) was greater than at Year 2 (50%). There were no statistically significant differences in these spectral domain OCT features between eyes assigned to ranibizumab and bevacizumab in the clinical trial or among eyes assigned to the different dosing regimens (Table 3 and 4 [available at http://www.aaojournal.org], Figures 3A and 3B).
Figure 3.
Mean total thickness at the foveal center and 95% confidence interval for 552 patients in the Follow-up Study with values available from optical coherent tomography. A. Overall and by drug assigned in the clinical trial; Overall and by dosing regimen assigned in the clinical trial.
Figure 4.
Retinal thickness at the foveal center in 553 patients with values available from optical coherent tomography, by category over time.
Figure 5.
Percentage of eyes with fluid for 552 eyes in the Follow-up Study with values available from optical coherent tomography, over time.
Morphologic Outcomes from Fundus Photography and Angiography
Fundus photographs were available for 527 (81.4%) of the Follow-up Study patients and fluorescein angiograms were available for 467 (72.2%; Table 3). Fluorescein leakage was detected in 111 (24.5%) eyes. The mean area of the total neovascular lesion was 12.9 (11.4) mm2, an increase of 4.8 (8.8) mm2 from 2 years. Geographic atrophy was present in 213 eyes (41.4%), and was subfoveal in 85 (16.5%). Fibrotic scar was present in the foveal center in 93 (19.6%) of eyes and non-fibrotic scar in an additional 26 (5.5%) among 474 gradable eyes. The mean area of the total neovascular lesion was 2 mm2 greater in eyes assigned to ranibizumab in the clinical trial than in eyes assigned to bevacizumab (13.9 vs 11.9 mm2); however, the difference was not statistically significant (p=0.06). Percentages of eyes with fluorescein leakage and with geographic atrophy were similar between eyes assigned to ranibizumab and eyes assigned to bevacizumab. There were no statistically significant differences in these features on fundus photography and angiography among eyes assigned to the different dosing regimens (Table 4 [available at http://www.aaojournal.org]).
Safety Data
Deaths and serious medical events occurring after 2 years are displayed in Table 5. There were 203 (18.6%) of 1090 patients who survived to 2 years but died before a Follow-up Study visit. Among 555 patients originally assigned to ranibizumab, 42 (7.6%) had an arteriothrombotic event compared with 24 (4.5%) of patients originally assigned to bevacizumab (p=0.04). Otherwise, there were no statistically significant differences in the type of serious medical events between drug or dosing regimen groups (Table 6 [available at http://www.aaojournal.org]).
Table 5.
Serious Medical Events between Year 2 and the Follow-up Study Visit by Drug Assigned in the Clinical Trial
| All (N=1090) | Drug Assigned in the Clinical Trial | ||||||
|---|---|---|---|---|---|---|---|
| Ranibizumab (N=555) | Bevacizumab (N=535) | ||||||
|
| |||||||
| Serious Medical Event | n | (%) | n | (%) | n | (%) | P§ |
| Death-all causes | 203 | (18.6) | 101 | (18.2) | 102 | (19.1) | 0.76 |
| Arteriothrombotic events | 66 | (6.1) | 42 | (7.6) | 24 | (4.5) | 0.04 |
| Venous thrombotic events | 9 | (0.8) | 4 | (0.7) | 5 | (0.9) | 0.75 |
| Hypertension | 27 | (2.5) | 13 | (2.3) | 14 | (2.6) | 0.85 |
| One or more serious medical events | 533 | (48.9) | 273 | (49.2) | 260 | (48.6) | 0.86 |
| Previously associated with anti-VEGF treatment‡ | 177 | (16.2) | 90 | (16.2) | 77 | (14.4) | 0.15 |
| MedDRA† system organ class** | |||||||
| Cardiac disorders | 120 | (11.0) | 62 | (11.2) | 58 | (10.8) | 0.92 |
| Infections | 75 | (6.9) | 32 | (5.8) | 43 | (8.0) | 0.15 |
| Nervous system disorders | 82 | (7.5) | 44 | (7.9) | 38 | (7.1) | 0.65 |
| Injury and procedural complications | 57 | (5.2) | 33 | (5.9) | 24 | (4.5) | 0.34 |
| Neoplasms benign and malignant | 97 | (8.9) | 48 | (8.6) | 49 | (9.2) | 0.83 |
| Gastrointestinal disorders | 36 | (3.3) | 20 | (3.6) | 16 | (3.0) | 0.61 |
Fisher’s exact test
Arteriothrombotic events, systemic hemorrhage, congestive heart failure, venous thrombotic events, hypertension, vascular death
Medical Dictionary for Regulatory Activities
DISCUSSION
The randomized clinical trials that established the efficacy of ranibizumab, bevacizumab, and aflibercept demonstrated that anti-VEGF therapy for neovascular AMD improved visual acuity on average by 1 to 2 lines through 2 years.1,2,6–14,16,17 The CATT Follow-up Study provides long-term follow-up (mean 5.5 years) on 70.8% of survivors. Mean visual acuity declined to 3 letters worse than at baseline and 11 letters worse than at the 2 years. This decrease in vision was accompanied by expansion of the size of the total neovascular complex composed of neovascularization, scarring, and atrophy and by persistence of fluid on OCT. Despite these morphologic changes, 50% of CATT Follow-up Study patients had a visual acuity 20/40 or better, while only 20% had visual acuity 20/200 or worse. These results emphasize both the tremendous advances over the past 15 years in preserving vision for a large proportion of patients as well as the limitations of current treatment.
The characteristics of the CATT patients who returned for the Follow-up Study are important to interpret the 5-year results. Overall, 71% of living patients from the original clinical trial population returned. On average, these patients were 2 years younger, had visual acuity that was 3 letters better at baseline, and had visual acuity that was 5 letters better at 2 years than patients who did not return. Study eyes received an average of 15.4 injections after release from the clinical trial protocol and most received regular care by their CATT ophthalmologist, even if not receiving frequent treatment. Among the group not returning were patients who dropped out of the clinical trial, were too ill to participate, moved out of the area, or refused to return. Thus, the Follow-up Study results are likely better than would have been observed if 100% of CATT patients had returned. In addition, some of the Follow-up Study participants did not have an OCT scan (14%), color photographs (19%), or a fluorescein angiogram (28%), eroding the generalizability of the Follow-up Study results on morphological outcomes.
Similarly, the long-term outcomes of patients treated with anti-VEGF drugs reported from other studies (discussed below) are likely better than if all patients originally identified had been observed. The magnitude of the overestimation is related to the degree of selection of patients for study and the percentage of patients lost to follow-up. In the only other extended follow-up study of patients enrolled in a key randomized clinical trial for an anti-VEGF drug, participants were eligible for the HORIZON study only if their ophthalmologist believed that further treatment with ranibizumab beyond the 2-year clinical trial period would be beneficial.19 Comparison of participants to non-participants in this cohort showed that visual acuity and lesion characteristics were better for participants, and only 388 (65%) of these selected 600 participants had 4-year follow-up. Several large-scale retrospective or registry studies have reported 4- and 5-year outcomes, but as demonstrated in a retrospective review of patients in Australia, patients who stop returning for care often do so soon after losing vision, so that patients with better vision are over-represented in these studies.18,20–24
The CATT Follow-up Study finding of 50% of patients with VA of 20/40 or better at 5 years and nearly 10% with VA 20/20 or better is remarkable when one considers the visual acuity outcomes in neovascular AMD prior to the to the development of anti-VEGF treatment. Two years after diagnosis, fewer than 10% of patients retained vision of 20/40 or better with no treatment and fewer than 15% of patients treated with photodynamic therapy retained 20/40 or better.31–33 Visual acuity decreased to 20/200 or worse at 2 years in 45% to 75% of patients with no treatment and in 30% to 40% with photodynamic therapy, compared to 20% at 5 years in the CATT Follow-up Study.
In CATT and all randomized clinical trials of anti-VEGF treatment for neovascular AMD, most of the improvement in mean visual acuity from baseline occurred within the first 3 to 6 months with little erosion of the benefit through 2 years when a fixed schedule of monthly (ranibizumab, bevacizumab, aflibercept) or bi-monthly (aflibercept) treatment was maintained.1,7,9,17,34 In CATT, patients who switched at 1 year from a monthly to a PRN dosing regimen received 5 to 6 injections on average and experienced a mean visual acuity decrease of 2 to 3 letters over the second year.7 During the 3.5 year period after release from the CATT protocol, patients received 4 to 5 injections per year on average and the mean visual acuity decreased an additional 11 letters to 59 letters (20/63). Similarly, in HORIZON, mean visual acuity declined by 7 letters to 20/80 with total 4 injections on average during the 2 years following exit from the formal clinical trials.19 In the Australian retrospective review, the mean number of injections over 5 years was 25 with mean visual acuity decreasing to 20/63.20 In contrast, the mean number of injections was 11 over 5 years in the Pan-American Study and the mean visual acuity at 5 years was 20/250.18 Thus, more frequent treatment, both in the initial 2 years and in later years appears associated with better long-term outcomes, and many patients require treatment through 5 years and beyond. This observation is in distinct contrast to the experience of treating diabetic macular edema with anti-VEGF therapy where the majority of patients do not require treatment beyond 3 years. In Diabetic Retinopathy Clinical Research Network Protocol I clinical trial, a mean of 8 or 9 injections were given in Year 1, decreasing to 2 or 3 in Year 2, to 1 or 2 in Year 3, and to 0 or 1 in Year 4, depending on treatment assignment.35
The processes responsible for the decrease in vision in CATT and other studies are multiple but appear to be related to an increase in the proportion patients with an abnormally thin retina (<120 microns), an increase in prevalence of geographic atrophy, and a substantial increase in lesion size. We previously reported that retinal thinning to <120 microns was associated with worse VA outcomes at 1 and 2 years.36,37 The proportion of eyes with an abnormally thin retina increased from 22% at the end of Year 2 to 36% at 5 years.37 We also previously reported that the proportion of eyes with geographic atrophy was 20% at 2 years and this proportion increased to 41% at 5 years, with an increase in subfoveal geographic atrophy from 6% to 17%.7,37 Worse VA outcomes have also been associated with increased lesion size, and in the Follow-up Study, mean lesion size increased more than 50% over the 3.5 year period (Table 3). These data highlight the need for agents that can prevent or minimize geographic atrophy and expansion of the total neovascular lesion.
The specific contribution of persistent fluid to long-term vision loss is unclear. The proportion of eyes with fluid decreased the most during the first year of treatment, but remained relatively unchanged throughout the remaining 4 years of follow up. More than 70% of eyes demonstrated intraretinal, subretinal, or sub-RPE fluid as determined by the OCT Reading Center throughout the study (Figure 5). Since the elimination of fluid is the primary goal at most treatment visits and almost no patients received treatment at every visit, it is reasonable to assume that the amount of fluid was frequently small and not detected by the ophthalmologist or was tolerated because of stable vision. On a cross-sectional basis, the presence of intraretinal fluid is associated with worse visual acuity during anti-VEGF treatment while the presence of subretinal fluid is associated with better visual acuity.36–38 Further studies to quantify the amount and location of residual fluid and to assess their impact on visual acuity are warranted.
During CATT, both the use of ranibizumab and monthly treatment were associated with an increased rate of development of geographic atrophy. At the end of Year 2, eyes treated with ranibizumab had a higher incidence (21%) of geographic atrophy than eyes treated with bevacizumab (17%; p=0.02).7 However, in the IVAN study, the incidence was similar in eyes treated with ranibizumab (28%) and with bevacizumab (31%; p=0.46), decreasing the likelihood of a true effect of ranibizumab on development of geographic atrophy.9 The association of monthly treatment with an increased rate of development of geographic atrophy was more consistent. At the end of Year 2 of CATT, eyes that received monthly treatment were more likely to have developed geographic atrophy than those treated with PRN therapy (24% vs 15%, p=0.003).7 In the IVAN study, 34% of eyes that received continuous (monthly) treatment developed geographic atrophy as compared with 26% in the discontinuous (PRN) group (p=0.03).9 In the HARBOR trial, eyes that received monthly ranibizumab had a higher incidence of geographic atrophy when compared with PRN treatment (HR, 1.3; 95% CI, 1.0–1.7).29 After release from the clinical trial at 2 years, very few patients continued monthly treatment and most were treated with at least 1 additional anti-VEGF drug that was different from their original treatment assignment. When examining the 5-year data for evidence of a residual drug or dosing effect on the development of geographic atrophy, there was still a higher proportion (44%) of eyes originally assigned to ranibizumab with geographic atrophy than eyes assigned to bevacizumab (38%), and a higher proportion (47%) of eyes assigned to monthly treatment for 2 years with geographic atrophy than eyes assigned to PRN treatment (40%). However, these differences were not statistically significant.
Although few patients remained on their originally assigned drug and dosing regimen beyond the 2-year period of the clinical trial, our study does allow assessment as to whether or not the drugs and dosing regimens used during the first 2 years led to any detectable outcome differences at 5 years. At the end of 2 years of treatment in the clinical trial, mean VA was 70 letters (20/40) and there was no statistically significant difference in mean visual acuity between eyes originally assigned to ranibizumab and eyes originally assigned to bevacizumab. Over the next 3.5 years of follow-up, patients originally assigned to ranibizumab lost more vision (−13 letters) than those originally assigned to bevacizumab (−9 letters; p=0.008; Figure 2A). The reasons for the decline are unclear, but it is clear that 2 years of initial therapy with bevacizumab and the accompanying lesser degree of reduction in fluid and retinal thickness did not compromise long-term visual acuity outcomes relative to ranibizumab, as some had speculated. There were no obvious differences in visual acuity outcomes at 5 years between patients who were treated monthly for 2 years versus those treated PRN for 2 years.
With most patients changing drugs over time, the ability to identify differential safety effects of the 2 drugs is compromised. During the period between the end of the clinical trial and the Follow-up Study visit, more patients originally assigned to ranibizumab had arteriothrombotic events than patients assigned to bevacizumab (7.6% versus 4.5%; p=0.04). However, during the 2 years of the clinical trial, the proportion of patient with these events was nearly equal with 4.7% of ranibizumab-treated patients and 5.0% of bevacizumab-treated patients having an event (p=0.62). Because of the absence of any difference when the history of drug exposure was certain we do not believe that the difference in events observed when a large portion of patients were not receiving ranibizumab are meaningful. Otherwise, we did not identify any statistically significant differences between groups based on the initially assigned drugs with respect to death or serious medical events. Overall concerns about the relative safety of bevacizumab and ranibizumab when treating patients with neovascular AMD have largely been assuaged by the results of 2 Cochrane comprehensive meta-analyses clinical trials comparing ranibizumab and bevacizumab.15,40
In summary, the CATT Follow-up Study provides the most complete follow up reported to date on the long-term outcomes for the treatment of neovascular AMD with anti-VEGF drugs. The original trial was designed to assess differences between ranibizumab and bevacizumab as well as differences between monthly and PRN dosing. Because very few patients remained on their originally assigned drug or dosing schedule between the end of year 2 and follow-up at approximately 5 years, the CATT Follow-up study results provide information primarily on overall treatment outcomes with anti-VEGF drugs and limited information on effects of different drugs and dosing regimens. Mean visual acuity at 5 years was 3 letters worse than baseline, highlighting an unmet need for further therapeutic advances. Still, 50% of patients were 20/40 or better and almost 10% were 20/20. These results would have been unimaginable in the era prior to the availability of anti-VEGF therapy.
Supplementary Material
Acknowledgments
Supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, and U10 EY017828 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services. The funding organization participated in the design and conduct of the study, data analysis and interpretation, and review of the manuscript.
Abbreviations and Acronyms
- AMD
age-related macular degeneration
- CATT
Comparison of Age-related Macular Degeneration Treatments Trials
- OCT
optical coherence tomography
- VA
visual acuity
- VEGF
vascular endothelial growth factor
Writing Committee
| Maureen G. Maguire, PhD | {University of Pennsylvania, Department of Ophthalmology} |
| Daniel F. Martin, MD | {Cleveland Clinic, Cole Eye Institute} |
| Gui-shuang Ying, PhD | {University of Pennsylvania, Department of Ophthalmology} |
| Glenn J. Jaffe, MD | {Duke University, Department of Ophthalmology} |
| Ebenezer Daniel, MBBS, PhD | {University of Pennsylvania, Department of Ophthalmology} |
| Juan E. Grunwald, MD | {University of Pennsylvania, Department of Ophthalmology} |
| Cynthia A. Toth | {Duke University, Department of Ophthalmology} |
| Frederick L. Ferris, 3rd,MD; | {National Eye Institute} |
| Stuart L. Fine, MD | {University of Colorado-Denver, Department of Ophthalmology} |
Footnotes
Part of this material is to be presented at the ARVO 2016 Annual Meeting.
ClinicalTrials.gov number NCT00593450
This article contains additional online-only material. The following should appear online-only: Tables 4 and 6, and Appendix.
Conflict of Interest Disclosure: Dr. Maguire reports personal fees from Roche/Genentech; Dr. Ying reports personal fees from Janssen R & D and Chengdu Kanghong Biotech Co; Dr. Jaffe reports personal fees from Alcon/Novartis, Neurotech, and Roche/Genentech; Dr. Toth grants from Genentech, Inc., personal fees from Alcon, Inc. and Thrombogenics, non-financial support from Bioptigen, and a patent unlicensed issued to Duke University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]; Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- 3.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128:1273–1279. doi: 10.1001/archophthalmol.2010.223. [DOI] [PubMed] [Google Scholar]
- 5.The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. Epub 2011 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The CATT Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: 2-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The IVAN Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration. One-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–67. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 9.Kodjikian L, Souied EH, Mimoun G, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL Noninferiority Randomized Trial. Ophthalmology. 2013;120:2300–9. doi: 10.1016/j.ophtha.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Krebs I, Schmetterer L, Boltz A, et al. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97:266–71. doi: 10.1136/bjophthalmol-2012-302391. [DOI] [PubMed] [Google Scholar]
- 11.Berg K, Pederson TR, Sandvik L, Bragadottir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122:146–52. doi: 10.1016/j.ophtha.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 12.Berg K, Hadzalic E, Gjertsen I, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the Lucentis compared to Avastin study treat-and-extend protocol: Two-year results. Ophthalmology. 2016;123:51–9. doi: 10.1016/j.ophtha.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Schauwvlieghe A-SM, Dijkman G, Hooymans JM, et al. Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age-related macular degeneration. BRAMD. Invest Ophthalmol Vis Sci. 2014;55:ARVO E-Abstract 870. doi: 10.1371/journal.pone.0153052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon SD, Lindsley KB, Krzystolik MG, Vedula SS, Hawkins BS. Intravitreal bevacizumab versus ranibizumab for treatment of neovascular age-related macular degeneration: Findings from a Cochrane Systematic Review. Ophthalmology. 2016;123:70–7. doi: 10.1016/j.ophtha.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heier JS, Brown DM, Chong V, Korobelnik JF, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Arevalo JF, Lasave AF, Wu L, et al. Intravitreal bevacizumab for choroidal neovasculariation in age-related macular degeneration: 5-year results of the Pan-American Collaborative Retina Study Group. Retina. 2015 Nov 2; doi: 10.1097/IAE.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 18.Singer MA, Awh CC, Sadda S, et al. HORIZON: An open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–83. doi: 10.1016/j.ophtha.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Gillies MC, Campain A, Barthelmes D, et al. Long-Term Outcomes of Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmology. 2015;122:1837–45. doi: 10.1016/j.ophtha.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Peden MC, Suner IJ, Hammer ME, et al. Long-Term Outcomes in Eyes Receiving Fixed-Interval Dosing of Anti-Vascular Endothelial Growth Factor Agents for Wet Age-Related Macular Degeneration. Ophthalmology. 2015;122:803–8. doi: 10.1016/j.ophtha.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen A, Bloch SB, Fuchs S, et al. A 4-Year Longitudinal Study of 555 Patients Treated with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2013;120:2630–36. doi: 10.1016/j.ophtha.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON. A multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–9. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Zhu M, Chew JK, Broadhead GK, et al. Intravitreal Ranibizumab for neovascular Age-related macular degeneration in clinical practice: five-year treatment outcomes. Arch Clin Exp Ophthalmol. 2015;253:1217–25. doi: 10.1007/s00417-014-2799-8. [DOI] [PubMed] [Google Scholar]
- 24.Grunwald JE, Daniel E, Ying G-S, Pistilli M, Maguire MG, Alexander J, Whittock-Martin R, Parker CR, Sepielli K, Blodi BA, Martin DF the CATT Research Group. Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119:1634–41. doi: 10.1016/j.ophtha.2012.02.013. Epub 2012 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeCroos FC, Toth CA, Stinnett SS, Heydary CS, Burns R, Jaffe GJ for the CATT Research Group. Optical coherence tomography grading reproducibility during the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119:2549–57. doi: 10.1016/j.ophtha.2012.06.040. Epub 2012 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan A, Duker JS, Ko TH, et al. Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol. 2006;124:193–8. doi: 10.1001/archopht.124.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antiplatelet Trialists’ Collaboration. Collaborative overview of randomized trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 28.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–77. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 29.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–85. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 30.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age related macular degeneration: updated findings from two clinical trials. Arch Ophthalmol. 1993;111:1200–9. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- 31.Treatment of Age-related Macular Degeneration With Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials- TAP Report #2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- 32.Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: Two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization-Verteporfin in Photodynamic Therapy Report 2. Am J Ophthalmol. 2001;131:541–560. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 33.Brown DM, Michels M, Kaiser PK, et al. ANCHOR Study Group. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two- year results of the ANCHOR Study. Ophthalmology. 2009;116:57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122:375–381. doi: 10.1016/j.ophtha.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffe GJ, Martin DF, Toth CA, et al. Macular morphology and visual acuity in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2013;120:1860–70. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma S, Toth CA, Daniel E, Grunwald JE, Maguire MG, Ying G-S, Huang J, Martin DF, Jaffe GJ the CATT Research Group. Macular morphology and visual acuity in the second year of the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2016 Jan 9; doi: 10.1016/j.ophtha.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt-Erfurth U, Waldstein SM, Deak G-G, Kundi M, Simader C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss int treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122:822–832. doi: 10.1016/j.ophtha.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Holz FG, Tuomi L, Ding B, Hopkins JJ. Development of atrophy in neovascular AMD treated with ranibizumab in the HARBOR study. Invest Ophthalmol Vis Sci. 2015;56:ARVO E- Abstract 890. [Google Scholar]
- 39.Moja L, Lucenteforte E, Kwag K, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014 Sep 15;9:CD011230. doi: 10.1002/14651858.CD011230.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.