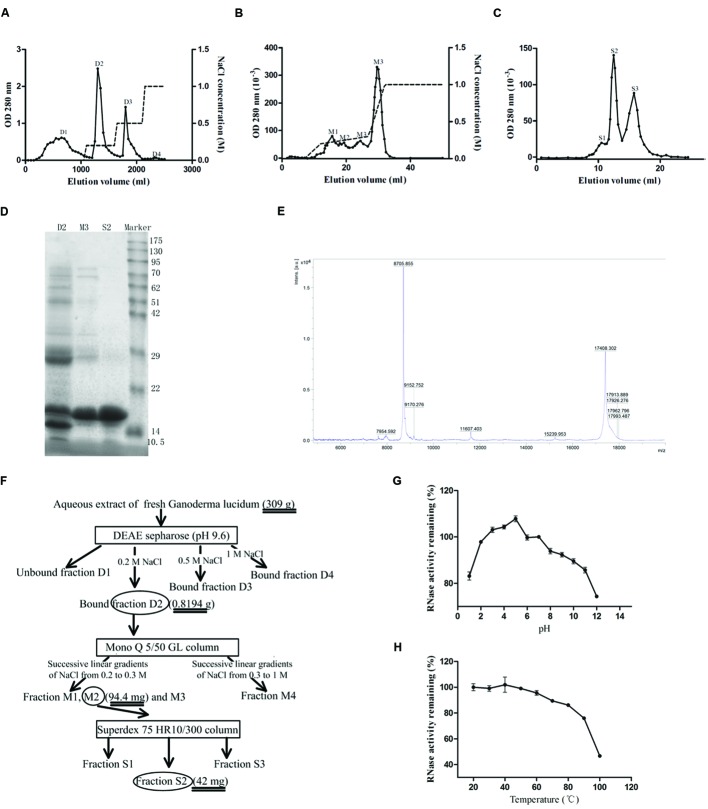
FIGURE 1.
Isolation of GLR. (A) Aqueous extract of Ganoderma lucidum was first loaded on a DEAE-Sepharose column. The adsorbed fractions were obtained by eluting the column with different concentrations of NaCl (represented by the dashed lines). Peak D2 containing the highest ribonuclease activity was collected. (B) Adsorbed peak D2 was subsequently loaded on a Mono Q column and the adsorbed fractions were eluted with three successive linear gradients of NaCl (0-0.2 M in 5 ml, 0.2-0.3 M in 15 ml, and 0.3-1 M in 5 ml). (C) The only peak with ribonuclease activity, M3, collected in the previous step was subjected to Superdex 75 10/300 GL column to yield purified RNase (peak S2). (D) SDS-PAGE showing purity and molecular weight of GLR (peak S2). From the left to the right, the lanes represented the bound fraction D2 from DEAE-Sepharose, the bound fraction M3 from Mono Q, fraction S2 from Superdex 75 and molecular weight marker proteins, respectively. (E) Mass spectrometry results indicated that the acquired S2 fraction had a molecular weight of 17408.302 kDa. (F) Schematic representation of the chromatographic steps used for the purification. Square frame and ellipse, respectively, represented the applied column and collected fractions. (G) pH stability of GLR was measured after incubation in buffers of different pH values for 30 min at room temperature. (H) Thermal stability of GLR was tested after incubation at different temperatures. The remaining ribonuclease activity of treated GLR in (G) and (H) was tested by using yeast tRNA as substrate (n = 3).