Figure 1.
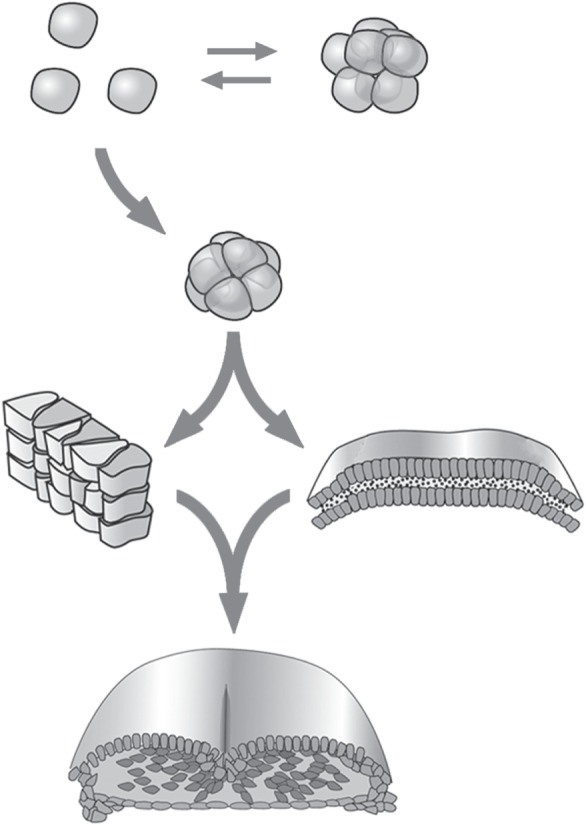
Major morphological transitions in the early history of the animals mediated by the emergence of novel biogeneric material properties. Ancestral unicellular holozoans (top row) had adhesive protocadherins and other cell surface molecules that permitted some species to form transient colonies. Certain subpopulations of these cells acquired DNA sequences specifying both Wnt, a secreted protein that induces A/B polarization, and the cytoskeleton-binding domain of the animal-specific classical cadherins. Together, these molecular novelties acted in cell aggregates to coordinate cell–cell adhesion with intracellular mechanics, constituting these clusters as cohesive ‘liquid tissues’ (second row), with the capacity to form lumens and undergo transient multilayering, forming body plans with features of present-day sponges and placozoans. Among these early emerging metazoans, some further acquired the capacity for tissue reshaping, such as elongation by convergent extension (third row, left), based on the PCP pathway elicited also by Wnt, but using novel mediators such as Vang/Stbm. Some of these organisms also acquired the ability to form stably layered sheet-like tissues (third row, right) and hence epithelial appendages, due to the presence of the novel enzyme peroxidasin which cross-links type IV collagen into a stiff, flexible basal lamina (stippling). Both PCP and a basal lamina are present in all extant eumetazoans, the morphologically simplest of which, the ctenophores and the cnidarians, are referred to together as ‘diploblasts,’ although their phylogenetic affinity is obscure. In some diploblastic forms, novel extracellular matrix molecules (galectins, TSR superfamily proteins, fibronectin) were acquired that elicited epithelial–mesenchymal transformation (EMT) in one or another of the two basic tissue layers during development, leading to an intermediate layer, the mesoblast, constituting the resulting animals as ‘triploblasts’ (fourth row; a developing bird embryo is represented). All of these triploblastic forms are bilaterally symmetric during at least part of their life cycles, possibly owing to the geometrical constraints of three-layered development, leading them to also be referred to as ‘bilaterians’. The phylogenetic affinities among many triploblastic groups and their genealogical relationships to extant diploblasts are unclear. A generalization that can be derived from this perspective is that certain major animal body plan categories have unambiguous morphological hallmarks that can be directly attributed to the material properties of their constituent tissues. These material properties, in turn, often depend straightforwardly on the consequences of the mobilization of new physical forces and effects by the products of novel genes [3,4].
