Abstract
Neurotransmitter degradation has been proposed to cause the accumulation of neurotoxic metabolites. The metabolism of these metabolites involves aldehyde dehydrogenase 2 (ALDH2). The Asian-specific single nucleotide polymorphism rs671 causes reduced enzyme activity. This study aims to explore whether Parkinson’s disease (PD) patients with reduced ALDH2 activity owing to the rs671 polymorphism are at risk for neuropsychological impairments. A total of 139 PD patients were recruited. Each participant was assessed for medical characteristics and their ALDH2 genotype. The Mini-Mental State Examination (MMSE), the Clinical Dementia Rating Scale and the Frontal Behavioral Inventory were used to measure neuropsychological functions. We found that the MMSE scores were significantly lower in patients with inactive ALDH2 (U = 1873.5, p = 0.02). The presence of cognitive impairments was significantly more frequent in the inactive ALDH2 group (46.0%) than in the active ALDH2 group (26.3%) (χ2 = 5.886, p = 0.01). The inactive group showed significant deterioration in hobbies and exhibited more severe “disorganization” and “hyper-sexuality” behaviours. The additive effects of the allele on the development of cognitive impairments in PD patients may be an important finding that provides further insight into the pathogenic mechanism of cognitive dysfunction in PD.
Parkinson’s disease (PD) is a common and progressive neurodegenerative disease that affected 4.1 to 4.6 million people over the age of 50 in 2005, with the numbers expected to double over the next two decades1. PD is characterized pathologically by the loss of dopaminergic neurons in the substantia nigra and manifests clinically as resting tremors, rigidity, bradykinesia, and postural instability. Although motor symptoms are the main clinical features of PD, increasing evidence has shown that PD patients also have non-motor symptoms, such as neurocognitive dysfunction and neuropsychiatric symptoms.
Cognitive impairment is one of the most common and devastating non-motor symptoms of PD. One systematic review suggested that the point prevalence of dementia in PD was more than 30%2, and individuals with PD had a three-to-six-fold higher risk of developing dementia than people of the same age without PD3. Approximately 80–90% of PD patients ultimately develop dementia by the end of longer follow-up periods4. Impaired cognitive functioning in PD patients results in a lower quality of life5, higher mortality6, heavier caregiver burden, and higher health-related costs7. Moreover, evidence suggests that the cognitive deficits are more heterogeneous than previously appreciated8. Some studies have shown that cognitive dysfunction is most prominent in the domains of memory9 and executive function10 in PD patients and other studies have shown that impairments in attentional/executive tasks may not always be the predominant deficit in PD11. Visuospatial deficits can also sometimes be the most severe cognitive impairments. Some studies have suggested that mild cognitive impairments in PD patients with predominant posterior cortical dysfunctions may result in a more rapid progression to dementia associated with PD (PDD)12. These studies clearly show the heterogeneity of neurocognitive dysfunctions in PD patients. However, the reasons for this heterogeneity remain elusive.
Monoamine neurotransmitters including dopamine and norepinephrine are essential components for nervous system functions and have been shown to be important for cognition in numerous studies. The importance of dopamine for cognitive functions is evidenced by the fact that high-level cognitive deficits are common in many dopamine-related disorders, such as addiction13 and schizophrenia14. The norepinephrine system is also of great importance in cognition based on the results of numerous animal and human studies that have explored the effects of noradrenaline manipulation on attention, working memory, cognitive flexibility, response inhibition and emotional memory15. Dopamine and noradrenaline are metabolized by catechol-O-methyl transferase (COMT) or monoamine oxidase (MAO) to intermediate metabolites. If dopamine and noradrenaline are initially metabolized by MAO, the metabolites are 3,4-dihydroxyphenylacetaldehyde (DOPAL) and 3,4-dihydroxyphenylglycolaldehyde (DOPEGAL), respectively. These aldehyde metabolites need to be further metabolized by aldehyde dehydrogenase (ALDH). Growing evidence has suggested that the aldehyde metabolites DOPAL and DOPEGAL are neurotoxic, and their intraneuronal accumulation has been associated with neuronal cell death leading to neurodegeneration16,17,18,19,20.
There are 19 human aldehyde dehydrogenase isoforms with wide tissue distributions and localizations in all subcellular compartments, including the cytosol, mitochondria, endoplasmic reticulum, and nucleus21. DOPAL metabolism primarily occurs in the mitochondrial fraction. Aldehyde dehydrogenase 2 (ALDH2) is a mitochondrial isozyme that has been proposed to be responsible for DOPAL metabolism22,23,24. The metabolism of DOPEGAL by ALDH2 has also been proposed because the DOPEGAL metabolism by ALDH2 is significantly impaired in the presence of acetaldehyde. This finding suggests that these aldehyde metabolites compete with acetaldehyde for ALDH2 metabolism25. The ALDH2 single nucleotide polymorphism (SNP) rs671(A), which results in an amino acid change from glutamic acid to lysine at position 504 (Glu504Lys) in the ALDH2 enzyme, is present almost exclusively in Asian populations26. The rs671(GG)-encoded enzyme is the active form, whereas the enzyme encoded by the rs671(AG) or rs671(AA) polymorphism is the partially or completely inactive form, respectively, and carries reduced enzymatic activity27,28. This reduced enzymatic activity has been shown to cause differences in susceptibility to many diseases, such as Asian flush29 and a series of cancers30.
In view of the crucial role of ALDH2 in the metabolism of the neurotoxic metabolites in the monoamine neurotransmitter pathway and the possible differences in metabolite accumulation caused by the rs671 SNP, we explored whether the rs671 SNP caused differences in the cognitive functions of PD patients.
Results
A total of 76 patients with rs671(GG) and 63 patients with rs671(AG) and (AA) were included in the study. There were no significant differences in age, gender, education level, age of onset, disease duration, levodopa equivalent daily dose (LED), or disease severity (stage) between the two groups (Table 1).
Table 1. Demographic and Clinical Characteristics of the Study Groups.
| rs671(GG) (n = 76) |
rs671(AG) and (AA) (n = 63) |
Statistic | p Value | |||
|---|---|---|---|---|---|---|
| mean | SD | mean | SD | |||
| Age (years) | 62.99 | 9.628 | 64.33 | 9.125 | 2216.000 | 0.451 |
| Gender (female/male) | 23/53 | — | 25/38 | — | χ2 = 1.352 | 0.163 |
| Education (years) | 11.32 | 4.431 | 11.56 | 4.617 | U = 2294.5 | 0.665 |
| Age at Onset (years) | 56.16 | 9.607 | 57.43 | 8.840 | U = 2210.0 | 0.436 |
| Disease duration (years) | 6.828 | 4.605 | 6.904 | 6.308 | U = 2224.0 | 0.470 |
| Levodopa equivalent dose | 673.413 | 535.4 | 641.266 | 487.333 | U = 2253.0 | 0.755 |
| Hoehn and Yahr stage | 2.29 | 0.995 | 2.36 | 1.079 | U = 2021.5 | 0.723 |
| I | 23.9% | — | 28.8% | — | χ2 = 3.069 | 0.381 |
| II | 38.0% | — | 23.7% | — | ||
| III | 23.9% | — | 30.5% | — | ||
| IV | 14.1% | — | 16.9% | — | ||
Abbreviations: SD, standard deviation.
In terms of general cognitive functioning, there was a significant difference in the total Mini-Mental State Examination (MMSE) score between the patients with rs671(GG) and rs671(AG) and (AA) (27.24 and 25.73, respectively), with the latter showing a poorer performance (U = 1873.5, p = 0.026). Using the MMSE cut-off value for dementia proposed by the Movement Disorder Society (MDS), we found significant differences between the proportions of patients with dementia in the two groups. The presence of dementia was significantly more frequent in the rs671(AG) and (AA) group (46.0%) than in the rs671(GG) group (26.3%) (χ2 = 5.886, p = 0.012). (Fig. 1).
Figure 1. Percentage of patients with MMSE scores higher than or lower/equal to 26 as proposed by the Movement Disorder Society.
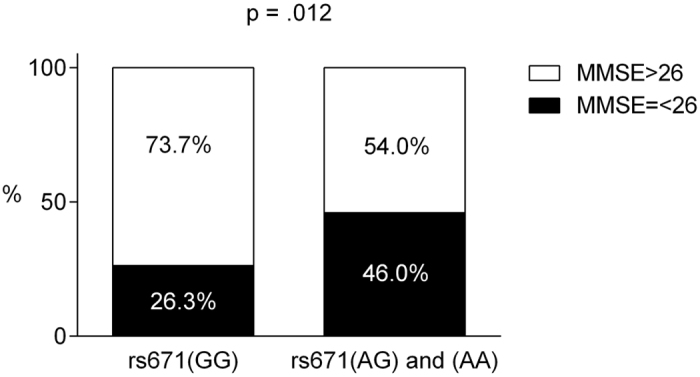
The presence of an MMSE lower than or equal to 26 was significantly more frequent in the rs671(AG) and (AA) group (46.0%) than the rs671(GG) group (26.3%) (p = 0.012).
The patients with rs671(GG) tended to score better on all Clinical Dementia Rating Scale (CDR) subscales; however, these differences were only significant for home hobbies (χ2 = 7.524, p = 0.023) (Table 2).
Table 2. Cognitive functions in the Study Groups.
| rs671(GG) (n = 76) |
rs671(AG) and (AA) (n = 63) |
Statistic | p Value | |||
|---|---|---|---|---|---|---|
| mean | SD | mean | SD | |||
| Mini-Mental State Examination | 27.24 | 3.506 | 25.73 | 4.505 | U = 1873.5 | 0.026 |
| Orientation | 9.53 | 0.973 | 8.97 | 1.814 | U = 2054.5 | 0.085 |
| Attention | 6.99 | 1.400 | 6.84 | 1.461 | U = 2260.5 | 0.540 |
| Memory | 2.08 | 1.030 | 1.70 | 1.159 | U = 1956.5 | 0.051 |
| Language | 4.86 | 0.423 | 4.65 | 0.600 | U = 1993.5 | 0.014 |
| Naming | 2.00 | 0.000 | 2.00 | 0.000 | U = 2394.0 | 1.00 |
| Repetition | 100% | 100% | — | — | ||
| Reading comprehension | 96.1% | 92.1% | χ2 = 1.011 | 0.261 | ||
| Make up a sentence | 89.5% | 73.0% | χ2 = 6.325 | 0.011 | ||
| Follow a 3-step command | 2.93 | 0.250 | 2.78 | 0.552 | U = 2128.5 | 0.042 |
| Construction | 81.6% | 77.8% | χ2 = 0.309 | 0.364 | ||
| Clinical Dementia Rating Scale | ||||||
| 0 | 31.6% | 33.3% | χ2 = 5.151 | 0.076 | ||
| 0.5 | 64.5% | 52.4% | ||||
| ≥1 | 3.9% | 14.3% | ||||
| CDR-Memory | ||||||
| 0 | 34.2% | 36.5% | χ2 = 2.872 | 0.238 | ||
| 0.5 | 47.4% | 34.9% | ||||
| ≥1 | 18.4% | 28.6% | ||||
| CDR-Orientation | ||||||
| 0 | 72.4% | 69.8% | χ2 = 2.649 | 0.266 | ||
| 0.5 | 22.4% | 17.5% | ||||
| ≥1 | 5.3% | 12.7% | ||||
| CDR-Judgement | ||||||
| 0 | 78.9% | 66.7% | χ2 = 4.271 | 0.118 | ||
| 0.5 | 17.1% | 20.6% | ||||
| ≥1 | 3.9% | 12.7% | ||||
| CDR-Community Affairs | ||||||
| 0 | 77.6% | 76.2% | χ2 = 1.941 | 0.379 | ||
| 0.5 | 17.1% | 12.7% | ||||
| ≥1 | 5.3% | 11.1% | ||||
| CDR-Home Hobbies | ||||||
| 0 | 78.9% | 79.4% | χ2 = 7.524 | 0.023 | ||
| 0.5 | 17.1% | 6.3% | ||||
| ≥1 | 3.9% | 14.3% | ||||
| CDR-Personal Care | ||||||
| 0 | 93.4% | 85.7% | χ2 = 2.259 | 0.111 | ||
| ≥1 | 6.6% | 14.3% | ||||
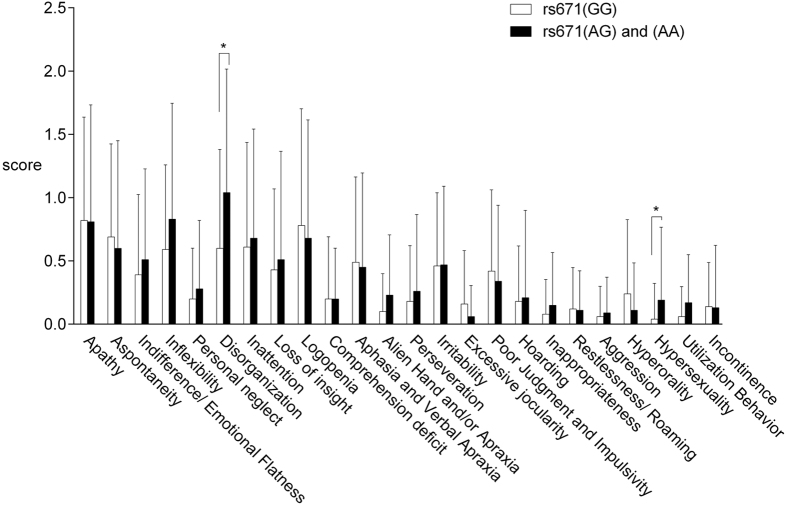
A total of 98 patients had a caregiver, and these family members completed the Frontal Behavioral Inventory (FBI). All of the caregivers were family members (60% of them were the patients’ spouses, 33% were the patients’ children, and 7% were the patient’s collateral relatives). PD patients with rs671(AG) and (AA) presented more frontal behavioral changes as evaluated by the FBI. When analysing the FBI sub-items, the patients with rs671(AG) and (AA) showed significantly higher scores for “disorganization” (U = 871.5, p = 0.018) and “hyper-sexuality” (U = 1050.0, p = 0.044). However, no significant difference was found in the total FBI scores (Fig. 2).
Figure 2. The means and standard deviations of each item on the Frontal behavioral Inventory in the 2 study groups.
Patients with rs671(AG) and (AA) showed significantly higher scores for “disorganization” (p = 0.018) and “hyper-sexuality” (p = 0.044).
Discussion
To the best of our knowledge, no studies have investigated the role of ALDH2 in the cognitive functions of PD patients. We conducted a cross-sectional study to investigate whether there were differences in these neuropsychological functions, including cognitive functioning and behavioural problems, in PD patients carrying different rs671 genotypes. By comparing the enzyme activity between the two study groups, which were satisfactorily matched for demographic and clinical features, we found a significant difference in the total MMSE score between patients with rs671(GG) and rs671(AG) and (AA), with the latter showing a poorer performance. The mean MMSE score of the patients with rs671(AG) and (AA) was 25.7, whereas the mean score of patients with rs671(GG) was 27.2. The MMSE was originally designed to provide a brief, standardized measurement of mental status. The originally recommended MMSE cut-off score of 24 provides good sensitivity for the detection of dementia31; however, several recent studies have suggested that this cut-off score may be too low, particularly for PD patients. The MDS Task Force proposed a practical diagnostic procedure32 and a diagnostic criteria33 for PD dementia in 2007, and an MMSE cut-off score of 26 was advised in the level I testing of the MDS diagnostic procedure. Using this recommended cut-off score, we found a significant difference in the proportions of patients with dementia between the two groups. The presence of dementia was significantly more frequent in the rs671(AG) and (AA) group (46%) than the rs671(GG) group (26%). This result shows that PD patients carrying the rs671(A) allele are at risk for the development of cognitive dysfunctions.
Cognitive impairments amongst PD patients are heterogeneous. Several studies have shown that the cognitive dysfunction of PD patients is most prominent in memory9, whereas other studies have shown that the deficit is most prominent in the attentional or executive functions domains10,34. In the current study, we found that patients with rs671(AG) and (AA) showed significantly worse performances in some MMSE subdomains in addition to the global MMSE score, including attention (i.e., follow a 3-step command) and language function (i.e., make up a sentence). Because the monoamine neurotransmitter system (including dopamine and noradrenaline) is closely related to attention, cognitive flexibility, and response inhibition in PD patients15,35, we propose that the loss-of-function SNP may cause the degeneration of dopaminergic and noradrenergic neurons through the accumulation of the neurotoxic metabolites (DOPAL and DOPEGAL) of dopamine and noradrenaline. The degeneration of dopaminergic and noradrenergic neurons results in the dysfunction of the attention function and frontal-related behaviour (e.g., disorganization and hyper-sexuality).
The memory function has traditionally been the key criterion for diagnosing dementia; however, growing evidence has shown the heterogeneous nature of cognitive deficits in patients with mild cognitive impairment (MCI) or dementia owing to various aetiologies36 (i.e., each cognitive domain plays an equally important role in the diagnosis of dementia). Many diagnostic criteria, such as the DSM-V37 and MDS criteria32, follow this concept. Several studies have shown that the cognitive profile of dementia in PD is different from that of Alzheimer’s disease (AD) and that this difference exists even in their precursor stages (i.e., the MCI stage). Hence, PD and AD are thought to show different cognitive impairment profiles. Our previous cross-sectional study used comprehensive neuropsychological testing to assess neurocognitive functioning in non-demented PD patients. We found that the cognitive dysfunctions in PD patients predominated in the anterior brain and that executive function was the most vulnerable domain in the cognitive decline process of PD34. Previous studies have shown that the ALDH2 SNP may increase the risk of AD development, the core clinical symptom of which is memory dysfunction38,39. The current study showed that PD patients with reduced ALDH2 activity had worse attention and language functions, which are not the cardinal dysfunction features of AD. This difference in the subdomains of the cognitive impairment profiles compared to typical AD suggests that the mechanisms underlying the cognitive impairments in PD owing to reduced ALDH2 activity are not simply a combination of the deficits of PD and AD. However, the underlying mechanisms for such differences could not be concluded in the current study. Further studies applying comprehensive performance-based neuropsychological test batteries to explore the detailed cognitive profiles caused by the ALDH2 SNP are needed.
In the current study, the severity of dementia symptoms (i.e., its ‘stage’) was quantified by the CDR. No significant difference was found between the two study groups in their global CDR scores, possibly because the CDR assessment tool has been traditionally used to measure the severity of AD-type dementia. Memory is the primary category in the rating process of the CDR’s global score, whereas the other 5 categories are secondary40. Thus, we cannot exclude the possibility that the lack of a significant difference between the two study groups may have been due to a lack of sensitivity in the CDR. Although the global CDR scores were similar between the two study groups, we found that the proportion of patients with impairments at home and in hobbies (one sub-domain in the CDR) was significantly different between the 2 groups. A higher percentage of patients carrying rs671(AG) or (AA) (14.3%) had reduced interests and impairments in home functioning than patients carrying rs671(GG) (3.9%). In rating each of these domains, the assessment focused on the patient’s cognitive ability to function in these areas. Limits of the participants in performing activities at home because of motor symptoms, physical frailty, or emotional status do not affect their scoring on the CDR. In other words, only impairments caused by cognitive dysfunction are rated. Therefore, we believe that the reported impairments of daily functioning may be due to the mental decline, such as initiation and organization. Maintaining the household (e.g., laundry, grocery shopping, taking out garbage, or basic home repair) and intellectual interests (e.g., entertaining, painting, or handicrafts) require individual initiation of the motion, planning and arrangement of the affairs day by day. The mental abilities mentioned above are components of executive function41, which is the most commonly impaired cognitive domain in PD34. Furthermore, when literate caregivers were asked to complete the FBI to measure the patient’s frontal-related behaviours, we found that patients with rs671(AG) and (AA) showed significantly more severe symptoms in their “disorganization” and “hyper-sexuality” behaviours. Disorganization, which is the lack of planning and sequencing, may be one of the earliest behavioural manifestations of the frontal dysexecutive phenomenon41,42. The findings suggest that ALDH2 inactivation may also result in the development of symptoms related to frontal lobe dysfunction.
ALDH2 is an important enzyme in the metabolism of monoamine neurotransmitters. Individuals carrying the rs671 A allele have reduced enzyme activity, which may lead to inefficient metabolism and accumulation of the neurotoxic metabolites of dopamine and noradrenaline and hence neuronal death and resultant cognitive dysfunction. Because levodopa therapy is one of the key medical treatments, it is possible that the additional dopamine converted from levodopa treatment can lead to increased accumulation of the neurotoxic metabolites and more rapid disease progression. Therefore, it is of great importance to study whether levodopa treatment causes more rapid progression of PD in patients carrying the rs671 A allele.
Although rs671 is an Asian-specific SNP, it is prevalent among Asians and is estimated to be present in more than 540 million individuals worldwide43. Recently, we found that cognitive dysfunction was more common in the Asian PD population than in Western PD patients44. Determining whether the ethnic difference is partially contributable to this SNP needs further study. However, the importance of ALDH2 in metabolizing the neurotoxic metabolites may not be limited to Asian populations. Genetic variation in ALDH2 among western population was recently shown to exacerbate the PD risk in subjects exposed to ALDH-inhibiting pesticides24. These findings suggest that dysregulation of ALDH2 activity through either genetic or environmental factors may be important for pathogenesis in PD patients from different ethnicities.
A potential limitation of the study was the lack of a comprehensive neuropsychological test battery that covered all of the cognitive domains to measure detailed cognitive functioning, particularly executive functioning. Although the MMSE is a well-known tool that is used to assess an individual’s global cognitive functioning, it is used as a screening tool for cognitive impairments. Second, the FBI is a caregiver-report questionnaire and hence may be biased by the responses provided by the caregiver. Therefore, the neurobehavioural problems may be more clearly and objectively evaluated with a semi-structured interview or other objective methods in future studies. Finally, we suggest that future studies should replicate the present findings in an independent PD population sample owing to the small sample size and the possibility of type I error.
In summary, to the best of our knowledge this is the first study to investigate the association between ALDH2 SNP rs671 and neuropsychological functioning in PD patients. The results indicate that PD patients with reduced ALDH2 activity are at risk for the development of cognitive dysfunctions and that these cognitive dysfunctions are different from those associated with AD. PD patients with reduced ALDH2 activity develop neurobehavioural problems significantly more frequently. These findings indicate that the ALDH2 SNP has an important impact on cognitive functioning in PD patients.
Methods
Participants
One hundred and thirty-nine patients who were diagnosed with idiopathic PD according to the United Kingdom PD Society Brain Bank clinical diagnostic criteria45 were enrolled in this study. The PD patients were recruited via referrals from neurologists at the Movement Disorders Centre. The exclusion criteria included the following: atypical features of parkinsonism, motor symptom onset before 50 years of age, illiteracy, a history of brain operation, and severe systemic disease.
Standard protocol approvals, registrations, and patient consent
All participants provided written informed consent prior to enrolment in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki. All study procedures were approved by the ethical research committee of National Taiwan University Hospital and Kaohsiung Medical University Hospital. All methods were performed in accordance with the approved guidelines. Each participant underwent a detailed interview to disclose demographic characteristics. Information on dopamine replacement therapy was recorded and the LED was calculated according to the recommendations of Tomlinson et al.46. Motor severity was quantified using the Hoehn and Yahr (H&Y) staging criteria47.
Neuropsychological assessment
All study participants underwent psychometric testing by a neuropsychologist experienced in the assessment of PD patients. Each patient was assessed while on medication.
General cognitive functioning was measured using the MMSE31, which has a maximum score of 30. The MMSE is composed of the following 11 subtests: temporal orientation (5 points), spatial orientation (5 points), immediate memory (3 points), attention/concentration (5 points), delayed recall (3 points), naming (2 points), verbal repetition (1 point), verbal comprehension (3 points), writing (1 point), reading a sentence (1 point), and constructional praxis (1 point).
Dementia severity was assessed using the CDR. The CDR is obtained through semi-structured interviews with patients and informants. Cognitive functioning is rated in the following 6 domains of functioning: memory, orientation, judgement and problem solving, community affairs, home and hobbies, and personal care. Each domain is rated on a 5-point scale of functioning as follows: 0, no impairment; 0.5, questionable impairment; 1, mild impairment; 2, moderate impairment; and 3, severe impairment (personal care is scored on a 4-point scale without a 0.5 rating available). The global CDR score was computed using the Washington University online algorithm40. The CDR demonstrates good psychometric properties48.
Frontal behavioral changes were measured using the FBI, which rates behavioural changes via caregiver interviews by a trained neuropsychologist using structured questions. The FBI was originally designed to measure behavioural changes in dementia patients and was useful in other disorders affecting frontal systems. The FBI has two subscales for negative and disinhibition behaviours and includes 24 items. Scoring of the answers is based on a 4-point scale of frequency ranging from “none” to “severe or most of the time”, wherein 3 reflects more severe frontal behavioral disorders; the scoring accounts for a total FBI score range of 0 to 7249.
Genotyping
Genetic testing was performed subsequent to the clinical and neuropsychological assessment. Genomic DNA was extracted from peripheral blood leukocytes of the participants with the Genomic DNA Extraction Kit (Geneaid, New Taipei City, Taiwan). ALDH2 rs671(A) genotyping was performed with TaqMan probes and the StepOnePlus™ system with the StepOne software™ (Applied Biosystems, Grand Island, NY, USA). The laboratory technician who performed the genotyping and read the genotype data was blinded to the patients’ clinical data.
Statistical analysis
Proportions were calculated for qualitative variables and means and standard deviations (SDs) were calculated for quantitative variables. The data were examined for normality and homogeneity of variance. The Chi-square test, t-test, and Mann-Whitney U test were used to examine group differences in nonparametric variables. Because less than 10% of patients had the rs671(AA) genotype, we combined the rs671(AG) and rs671(AA) genotypes for the data analysis. This combination is not illegitimate for the analysis of the acetaldehyde metabolic effects because the rs671(AA)-encoded enzyme is inactive and the rs671(AG)-encoded enzyme has an acetaldehyde metabolite rate that is only one-tenth that of rs671(GG)50. Statistical significance was determined when the probability value was less than 0.05. A commercially available software program (SPSS version 17.0; SPSS Inc., Chicago, IL, USA) was used for the statistical analyses.
Additional Information
How to cite this article: Yu, R.-L. et al. Aldehyde dehydrogenase 2 is associated with cognitive functions in patients with Parkinson’s disease. Sci. Rep. 6, 30424; doi: 10.1038/srep30424 (2016).
Acknowledgments
The authors are grateful to the participants involved in this study and for the grants from the Ministry of Science and Technology (MOST), Taipei, Taiwan (MOST 103-2410-H-006-120-MY2, MOST 103-2633-H-006-001, and MOST 104-2633-H-006 -001).
Footnotes
Author Contributions All authors fulfilled the criteria of authorship and no one who fulfilled the criteria was excluded. R.-L.Y. had the idea, designed the experiments, and produced the first draft of the manuscript. C.-H.T. and Y.-C.L. performed the experiments. C.-H.T. and R.-M.W. were involved in sample collection. R.-L.Y. and C.-H.T. performed literature searches and data analysis. R.-L.Y., C.-H.T. and R.-M.W. take responsibility for the interpretation of the results. All authors critically reviewed drafts and gave approval for the final version of this article. R.-M.W. accepts full responsibility for the work and controlled the decision to publish.
References
- Dorsey E. R. et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68, 384–386, doi: 10.1212/01.wnl.0000247740.47667.03 (2007). [DOI] [PubMed] [Google Scholar]
- Aarsland D., Zaccai J. & Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord 20, 1255–1263, doi: 10.1002/mds.20527 (2005). [DOI] [PubMed] [Google Scholar]
- Aarsland D. et al. Risk of dementia in Parkinson’s disease A community-based, prospective study. Neurology 56, 730–736 (2001). [DOI] [PubMed] [Google Scholar]
- Buter T. C. et al. Dementia and survival in Parkinson disease: a 12-year population study. Neurology 70, 1017–1022, doi: 10.1212/01.wnl.0000306632.43729.24 (2008). [DOI] [PubMed] [Google Scholar]
- Schrag A., Jahanshahi M. & Quinn N. What contributes to quality of life in patients with Parkinson’s disease? Journal of Neurology, Neurosurgery & Psychiatry 69, 308–312 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D., Beyer M. K. & Kurz M. W. Dementia in Parkinson’s disease. Curr Opin Neurol 21, 676–682, doi: 10.1097/WCO.0b013e3283168df0 (2008). [DOI] [PubMed] [Google Scholar]
- Vossius C., Larsen J. P., Janvin C. & Aarsland D. The economic impact of cognitive impairment in Parkinson’s disease. Mov Disord 26, 1541–1544, doi: 10.1002/mds.23661 (2011). [DOI] [PubMed] [Google Scholar]
- Kehagia A. A., Barker R. A. & Robbins T. W. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol 9, 1200–1213, doi: 10.1016/S1474-4422(10)70212-X (2010). [DOI] [PubMed] [Google Scholar]
- Aarsland D. et al. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 72, 1121–1126, doi: 10.1212/01.wnl.0000338632.00552.cb (2009). [DOI] [PubMed] [Google Scholar]
- Dujardin K. et al. Cognitive and SPECT characteristics predict progression of Parkinson’s disease in newly diagnosed patients. J Neurol 251, 1383–1392, doi: 10.1007/s00415-004-0549-2 (2004). [DOI] [PubMed] [Google Scholar]
- Kulisevsky J. & Pagonabarraga J. Cognitive impairment in Parkinson’s disease: tools for diagnosis and assessment. Mov Disord 24, 1103–1110, doi: 10.1002/mds.22506 (2009). [DOI] [PubMed] [Google Scholar]
- Aarsland D., Bronnick K. & Fladby T. Mild cognitive impairment in Parkinson’s disease. Current neurology and neuroscience reports 11, 371–378, doi: 10.1007/s11910-011-0203-1 (2011). [DOI] [PubMed] [Google Scholar]
- Dalley J. W., Everitt B. J. & Robbins T. W. Impulsivity, compulsivity, and top-down cognitive control. Neuron 69, 680–694, doi: 10.1016/j.neuron.2011.01.020 (2011). [DOI] [PubMed] [Google Scholar]
- Howes O. D. & Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull 35, 549–562, doi: 10.1093/schbul/sbp006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S. R. & Robbins T. W. Noradrenergic modulation of cognition: therapeutic implications. J Psychopharmacol 27, 694–718, doi: 10.1177/0269881113480988 (2013). [DOI] [PubMed] [Google Scholar]
- Li S. W., Lin T.-S., Minteer S. & Burke W. J. 3, 4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson’s disease pathogenesis. Molecular brain research 93, 1–7 (2001). [DOI] [PubMed] [Google Scholar]
- Burke W. J. et al. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology 25, 101–115, doi: 10.1016/S0161-813X(03)00090-1 (2004). [DOI] [PubMed] [Google Scholar]
- Kristal B. S. et al. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic Biol Med 30, 924–931 (2001). [DOI] [PubMed] [Google Scholar]
- Marchitti S. A., Deitrich R. A. & Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol Rev 59, 125–150, doi: 10.1124/pr.59.2.1 (2007). [DOI] [PubMed] [Google Scholar]
- Eisenhofer G., Kopin I. J. & Goldstein D. S. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56, 331–349, doi: 10.1124/pr.56.3.1 (2004). [DOI] [PubMed] [Google Scholar]
- Vasiliou V. & Nebert D. W. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Human genomics 2, 138–143 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamensdorf I. et al. 3,4-Dihydroxyphenylacetaldehyde potentiates the toxic effects of metabolic stress in PC12 cells. Brain Res 868, 191–201 (2000). [DOI] [PubMed] [Google Scholar]
- Fitzmaurice A. G. et al. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc Natl Acad Sci USA 110, 636–641, doi: 10.1073/pnas.1220399110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice A. G., Rhodes S. L., Cockburn M., Ritz B. & Bronstein J. M. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology 82, 419–426, doi: 10.1212/WNL.0000000000000083 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKerell A. D., Blatter E. E. & Pietruszko R. Human aldehyde dehydrogenase: kinetic identification of the isozyme for which biogenic aldehydes and acetaldehyde compete. Alcoholism: Clinical and Experimental Research 10, 266–270 (1986). [DOI] [PubMed] [Google Scholar]
- Flicek P. et al. Ensembl 2014. Nucleic Acids Res 42, D749–755, doi: 10.1093/nar/gkt1196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst F. M. et al. World Health Organization/International Society for Biomedical Research on Alcoholism study on state and trait markers of alcohol use and dependence: back to the future. Alcoholism: Clinical and Experimental Research 29, 1268–1275 (2005). [DOI] [PubMed] [Google Scholar]
- Crabb D. W., Edenberg H. J., Bosron W. F. & Li T.-K. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2 (2) allele is dominant. Journal of Clinical Investigation 83, 314 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Agarwal D. & Goedde H. Aldehyde dehydrogenase deficiency as cause of facial flushing reaction to alcohol in Japanese. The Lancet 318, 982 (1981). [DOI] [PubMed] [Google Scholar]
- Yokoyama A. et al. Alcohol and aldehyde dehydrogenase gene polymorphisms and oropharyngolaryngeal, esophageal and stomach cancers in Japanese alcoholics. Carcinogenesis 22, 433–439 (2001). [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E. & McHugh P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198 (1975). [DOI] [PubMed] [Google Scholar]
- Dubois B. et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord 22, 2314–2324, doi: 10.1002/mds.21844 (2007). [DOI] [PubMed] [Google Scholar]
- Emre M. et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22, 1689–1707; quiz 1837, doi: 10.1002/mds.21507 (2007). [DOI] [PubMed] [Google Scholar]
- Yu R. L. et al. Neuropsychological profile in patients with early stage of Parkinson’s disease in Taiwan. Parkinsonism & related disorders 18, 1067–1072, doi: 10.1016/j.parkreldis.2012.06.002 (2012). [DOI] [PubMed] [Google Scholar]
- Gratwicke J., Jahanshahi M. & Foltynie T. Parkinson’s disease dementia: a neural networks perspective. Brain, awv104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R. A. & Williams-Gray C. H. Mild cognitive impairment and Parkinson’s disease–something to remember. Journal of Parkinson’s disease 4, 651–656, doi: 10.3233/JPD-140427 (2014). [DOI] [PubMed] [Google Scholar]
- Association A. P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). (American Psychiatric Pub, 2013). [DOI] [PubMed] [Google Scholar]
- Hao P.-P., Chen Y.-G., Wang J.-L., Wang X. L. & Zhang Y. Meta-analysis of aldehyde dehydrogenase 2 gene polymorphism and Alzheimer’s disease in East Asians. Canadian Journal of Neurological Sciences/Journal Canadien des Sciences Neurologiques 38, 500–506 (2011). [DOI] [PubMed] [Google Scholar]
- Kamino K. et al. Deficiency in mitochondrial aldehyde dehydrogenase increases the risk for late-onset Alzheimer’s disease in the Japanese population. Biochem Biophys Res Commun 273, 192–196, doi: 10.1006/bbrc.2000.2923 (2000). [DOI] [PubMed] [Google Scholar]
- Morris J. C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414 (1993). [DOI] [PubMed] [Google Scholar]
- Elliott R. Executive functions and their disorders Imaging in clinical neuroscience. British medical bulletin 65, 49–59 (2003). [DOI] [PubMed] [Google Scholar]
- Stuss D. T. & Benson D. F. Neuropsychological studies of the frontal lobes. Psychol Bull 95, 3–28 (1984). [PubMed] [Google Scholar]
- Brooks P. J., Enoch M. A., Goldman D., Li T. K. & Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS medicine 6, e50, doi: 10.1371/journal.pmed.1000050 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R. L. et al. Cross‐Cultural Differences of the Non‐Motor Symptoms Studied by the Traditional Chinese Version of the International Parkinson and Movement Disorder Society–Unified Parkinson’s Disease Rating Scale. Movement Disorders Clinical Practice (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. J., Daniel S. E., Kilford L. & Lees A. J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55, 181–184 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25, 2649–2653, doi: 10.1002/mds.23429 (2010). [DOI] [PubMed] [Google Scholar]
- Hoehn M. M. & Yahr M. D. Parkinsonism: onset, progression and mortality. Neurology 17, 427–442 (1967). [DOI] [PubMed] [Google Scholar]
- Morris J. C. et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology 48, 1508–1510 (1997). [DOI] [PubMed] [Google Scholar]
- Kertesz A., Davidson W. & Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 24, 29–36 (1997). [DOI] [PubMed] [Google Scholar]
- Keung W. M. & Vallee B. L. Daidzin and its antidipsotropic analogs inhibit serotonin and dopamine metabolism in isolated mitochondria. Proc Natl Acad Sci USA 95, 2198–2203 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]