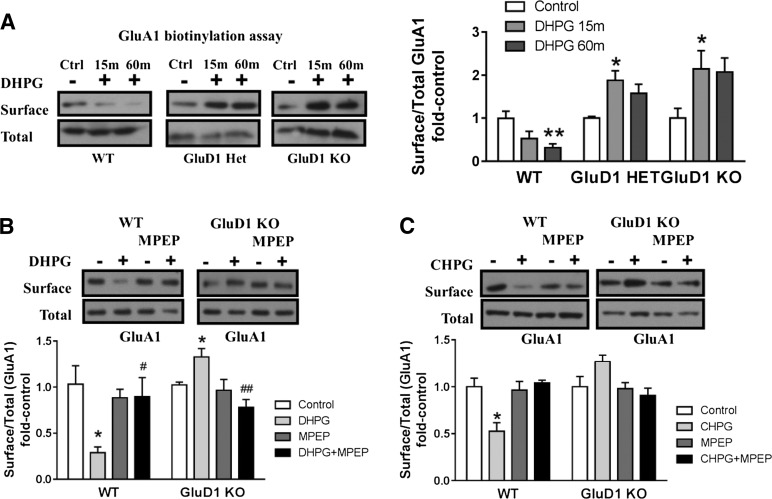
Fig. 4.
GluD1 deletion leads to abnormal mGlu5-mediated AMPA receptor internalization in acute hippocampal slices. (A) Biotinylation assays to detect changes in surface expression of GluA1 were performed in horizontal sections from wild-type (WT), GluD1 heterozygous, and GluD1 KO mice. DHPG (100 µM, 5 minutes in the presence of NMDA receptor antagonist) produced a reduction in surface GluA1 expression in wild-type sections (**P < 0.01, one-way ANOVA; N = 6–8 for each data point). In GluD1 KO, no reduction in the surface expression was observed, whereas a contrasting increase in surface GluD1 expression was observed after 15 minutes of DHPG treatment (*P < 0.05, one-way ANOVA; N = 7–9). Impaired internalization of surface GluA1 was also observed in GluD1 heterozygous mice (*P < 0.05, one-way ANOVA; N = 3). (B) DHPG treatment induced significant reduction in surface GluA1 in slices prepared from the wild type (*P < 0.05, unpaired t test compared with respective control) and an opposing increase in surface GluA1 expression in GluD1 KO animals (*P < 0.05, unpaired t test compared with respective control). Pretreatment with mGlu5-specific antagonist MPEP inhibited GluA1 internalization in wild-type slices, indicating the requirement of mGlu5 in this effect (N = 4–5). A significant difference was also observed between DHPG and DHPG+MPEP in wild-type as well as GluD1 KO (#P < 0.05 and ##P < 0.01, unpaired t test), supporting the requirement of mGlu5 in the effects produced by DHPG. (C) mGlu5-selective agonist CHPG leads to internalization of GluA1 in wild-type slices (*P < 0.05, unpaired t test), which was blocked by MPEP treatment. However, CHPG application failed to induce GluA1 internalization in GluD1 KO (N = 3). In each case, treatment groups were normalized to sham control of the respective genotype. Ctrl, control; Het, heterozygous.